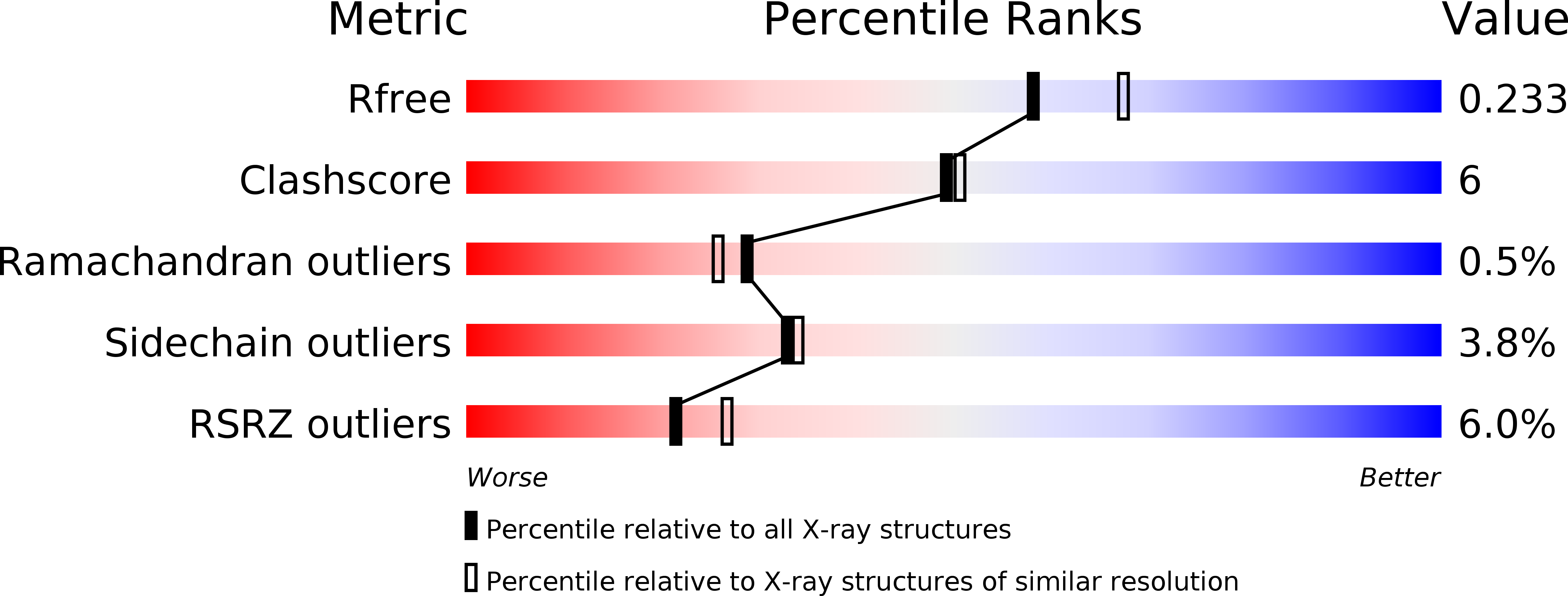
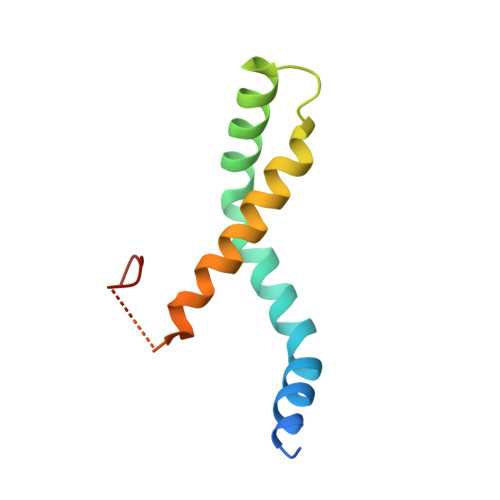
Structural and Functional Characterization of a Cell Cycle Associated Hdac1/2 Complex Reveals the Structural Basis for Complex Assembly and Nucleosome Targeting.
Itoh, T., Fairall, L., Muskett, F.W., Milano, C.P., Watson, P.J., Arnaudo, N., Saleh, A., Millard, C.J., El-Mezgueldi, M., Martino, F., Schwabe, J.W.R.(2015) Nucleic Acids Res 43: 2033
- PubMed: 25653165
- DOI: https://doi.org/10.1093/nar/gkv068
- Primary Citation of Related Structures:
2MWI, 4D6K - PubMed Abstract:
Recent proteomic studies have identified a novel histone deacetylase complex that is upregulated during mitosis and is associated with cyclin A. This complex is conserved from nematodes to man and contains histone deacetylases 1 and 2, the MIDEAS corepressor protein and a protein called DNTTIP1 whose function was hitherto poorly understood. Here, we report the structures of two domains from DNTTIP1. The amino-terminal region forms a tight dimerization domain with a novel structural fold that interacts with and mediates assembly of the HDAC1:MIDEAS complex. The carboxy-terminal domain of DNTTIP1 has a structure related to the SKI/SNO/DAC domain, despite lacking obvious sequence homology. We show that this domain in DNTTIP1 mediates interaction with both DNA and nucleosomes. Thus, DNTTIP1 acts as a dimeric chromatin binding module in the HDAC1:MIDEAS corepressor complex.
Organizational Affiliation:
Henry Wellcome Laboratories of Structural Biology, Department of Biochemistry, University of Leicester, Lancaster Road, Leicester LE1 9HN, UK.