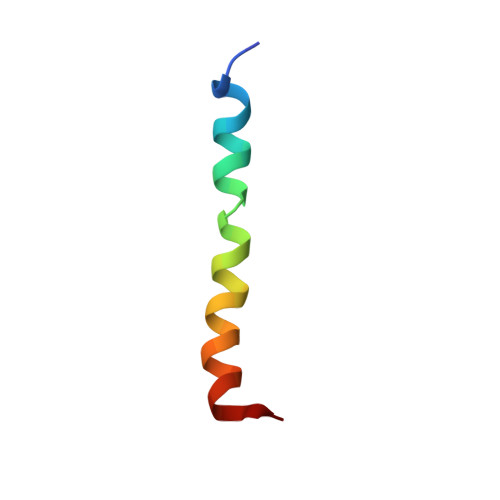
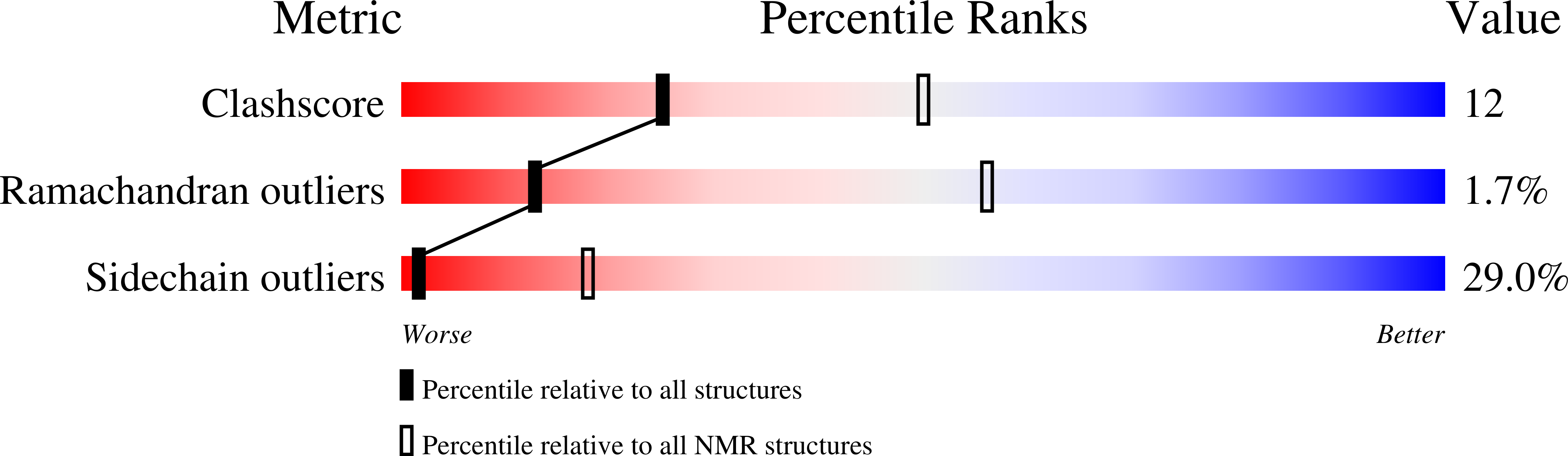Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers.
Mandala, V.S., McKay, M.J., Shcherbakov, A.A., Dregni, A.J., Kolocouris, A., Hong, M.(2020) Nat Struct Mol Biol 27: 1202-1208
- PubMed: 33177698
- DOI: https://doi.org/10.1038/s41594-020-00536-8
- Primary Citation of Related Structures:
7K3G - PubMed Abstract:
An essential protein of the SARS-CoV-2 virus, the envelope protein E, forms a homopentameric cation channel that is important for virus pathogenicity. Here we report a 2.1-Å structure and the drug-binding site of E's transmembrane domain (ETM), determined using solid-state NMR spectroscopy. In lipid bilayers that mimic the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) membrane, ETM forms a five-helix bundle surrounding a narrow pore. The protein deviates from the ideal α-helical geometry due to three phenylalanine residues, which stack within each helix and between helices. Together with valine and leucine interdigitation, these cause a dehydrated pore compared with the viroporins of influenza viruses and HIV. Hexamethylene amiloride binds the polar amino-terminal lumen, whereas acidic pH affects the carboxy-terminal conformation. Thus, the N- and C-terminal halves of this bipartite channel may interact with other viral and host proteins semi-independently. The structure sets the stage for designing E inhibitors as antiviral drugs.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA, USA.