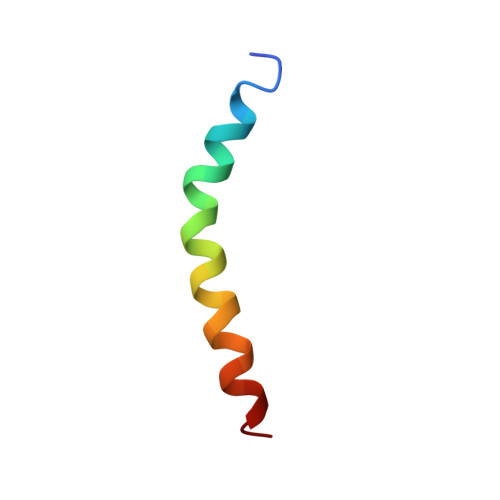
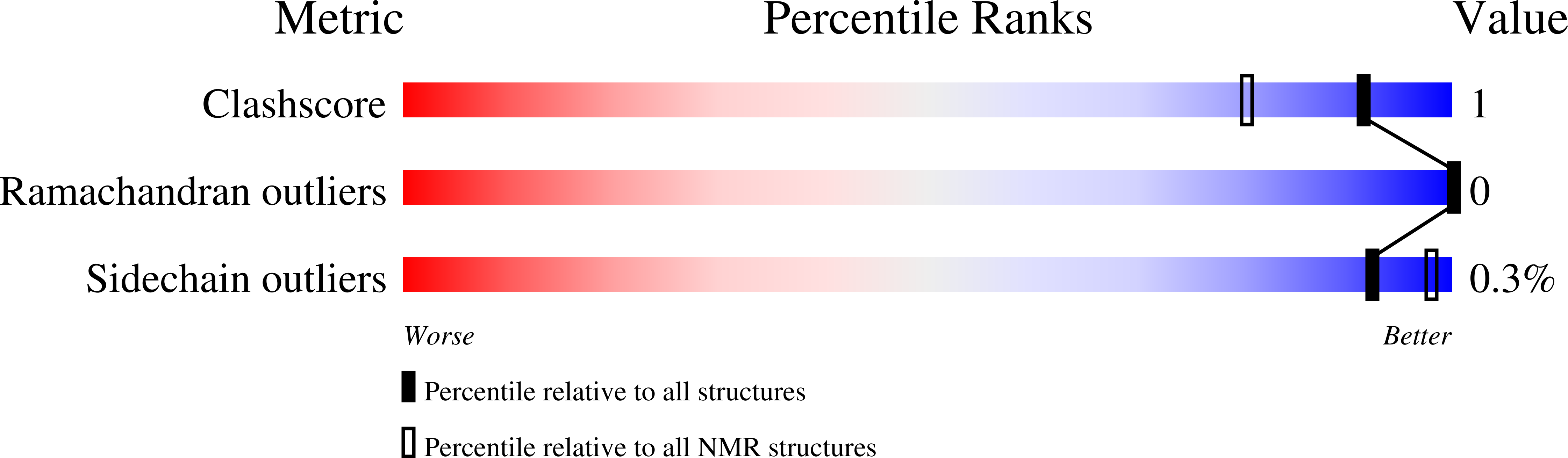Altered Hinge Conformations in APP Transmembrane Helix Mutants May Affect Enzyme-Substrate Interactions of gamma-Secretase.
Silber, M., Hitzenberger, M., Zacharias, M., Muhle-Goll, C.(2020) ACS Chem Neurosci 11: 4426-4433
- PubMed: 33232115
- DOI: https://doi.org/10.1021/acschemneuro.0c00640
- Primary Citation of Related Structures:
6YHF, 6YHI, 6YHO, 6YHP, 6YHX - PubMed Abstract:
Cleavage of substrates by γ-secretase is an inherently slow process where substrate-enzyme affinities cannot be broken down into specific sequence requirements in contrast to soluble proteases. Nevertheless, despite its apparent sequence tolerance single point mutations in amyloid precursor protein can severely affect cleavage efficiencies and change product line preferences. We have determined by NMR spectroscopy the structures of the transmembrane domain of amyloid precursor protein in TFE/water and compared it to that of four mutants: two FAD mutants, V44M and I45T, and the two diglycine hinge mutants, G38L and G38P. In accordance with previous publications, the transmembrane domain is composed of two helical segments connected by the diglycine hinge. Mutations alter kink angles and structural flexibility. Furthermore, to our surprise, we observe different, but specific mutual orientations of N- and C-terminal helical segments in the four mutants compared to the wildtype. We speculate that the observed orientations for G38L, G38P, V44M, and I45T lead to unfavorable interactions with γ-secretase exosites during substrate movement to the enzyme's active site in presenilin and/or for the accommodation into the substrate-binding cavity of presenilin.
Organizational Affiliation:
Institute for Biological Interfaces 4, Karlsruhe Institute of Technology, P.O. Box 3640, 76021 Karlsruhe, Germany.