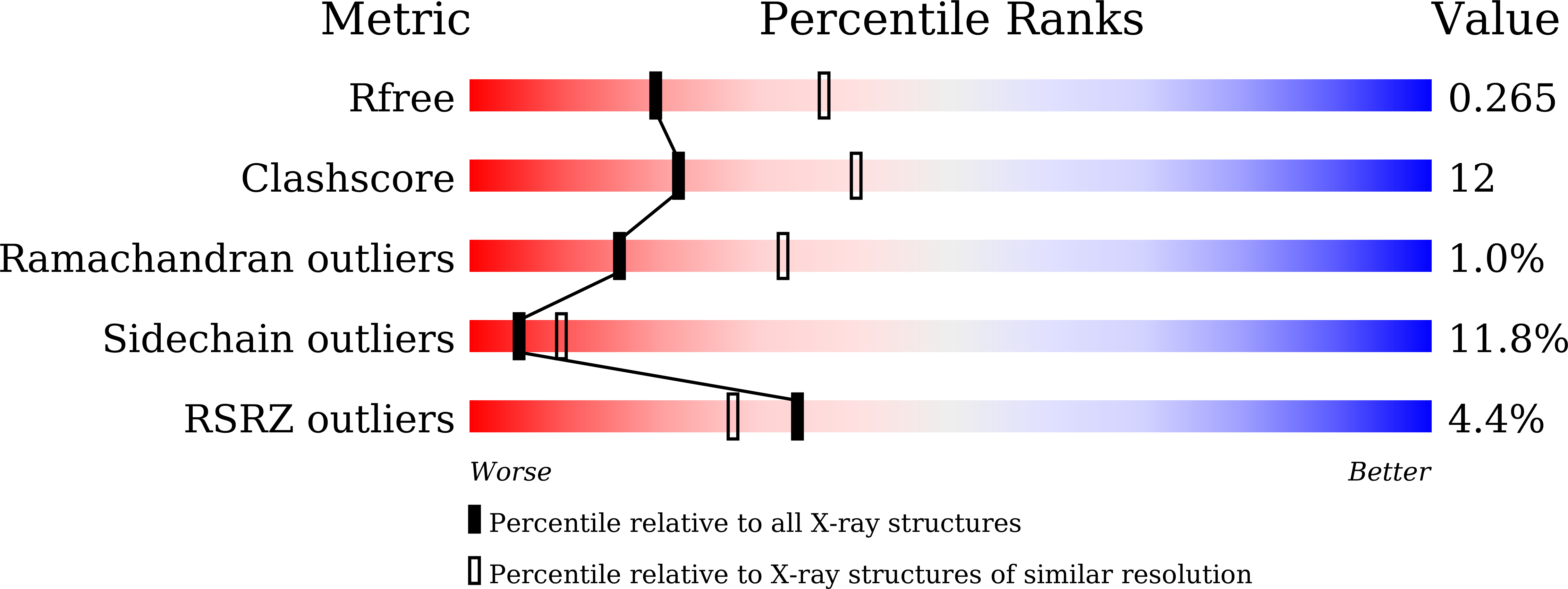
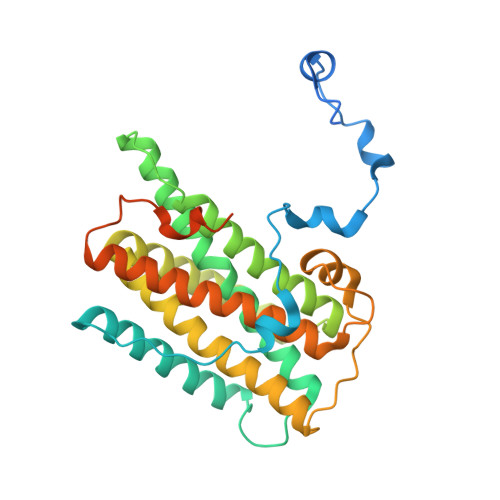
Insights into the ubiquinol/dioxygen binding and proton relay pathways of the alternative oxidase.
Shiba, T., Inaoka, D.K., Takahashi, G., Tsuge, C., Kido, Y., Young, L., Ueda, S., Balogun, E.O., Nara, T., Honma, T., Tanaka, A., Inoue, M., Saimoto, H., Harada, S., Moore, A.L., Kita, K.(2019) Biochim Biophys Acta Bioenerg 1860: 375-382
- PubMed: 30910528
- DOI: https://doi.org/10.1016/j.bbabio.2019.03.008
- Primary Citation of Related Structures:
5ZDP, 5ZDQ, 5ZDR - PubMed Abstract:
The alternative oxidase (AOX) is a monotopic diiron carboxylate protein which catalyzes the four-electron reduction of dioxygen to water by ubiquinol. Although we have recently determined the crystal structure of Trypanosoma brucei AOX (TAO) in the presence and absence of ascofuranone (AF) derivatives (which are potent mixed type inhibitors) the mechanism by which ubiquinol and dioxygen binds to TAO remain inconclusive. In this article, ferulenol was identified as the first competitive inhibitor of AOX which has been used to probe the binding of ubiquinol. Surface plasmon resonance reveals that AF is a quasi-irreversible inhibitor of TAO whilst ferulenol binding is completely reversible. The structure of the TAO-ferulenol complex, determined at 2.7 Å, provided insights into ubiquinol binding and has also identified a potential dioxygen molecule bound in a side-on conformation to the diiron center for the first time.
Organizational Affiliation:
Department of Applied Biology, Graduate School of Science and Technology, Kyoto Institute of Technology, Sakyo-ku, Matsugasaki, Kyoto 606-8585, Japan. Electronic address: tshiba@kit.ac.jp.