Structural basis for the acetylation of histone H3K9 and H3K27 mediated by the histone chaperone Vps75 inPneumocystis carinii.
Chen, Y., Zhang, Y., Ye, H., Dou, Y., Lu, D., Li, X., Limper, A.H., Han, J., Su, D.(2019) Signal Transduct Target Ther 4: 14-14
- PubMed: 31098304
- DOI: https://doi.org/10.1038/s41392-019-0047-8
- Primary Citation of Related Structures:
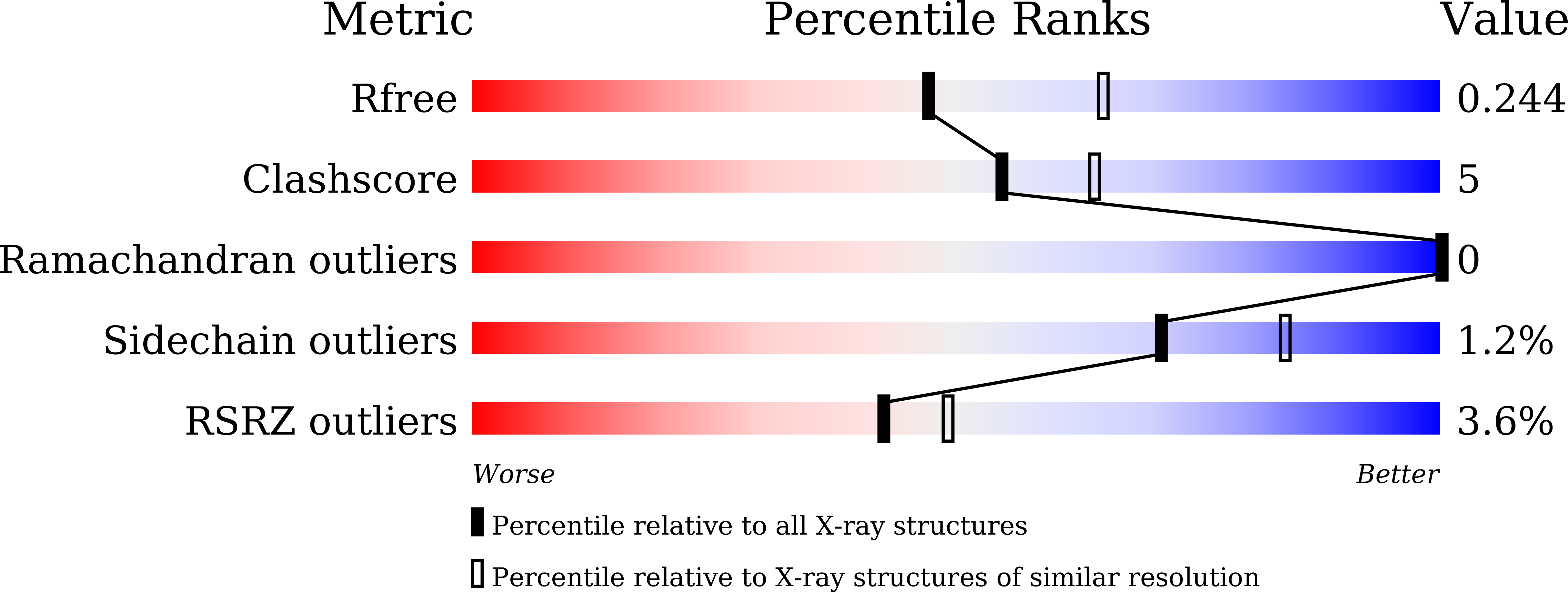
5YPS, 5ZB5 - PubMed Abstract:
Rtt109 is a histone acetyltransferase (HAT) that is a potential therapeutic target in conditioned pathogenic fungi Pneumocystis carinii (P. carinii) . The histone chaperone Vps75 can stimulate the Rtt109-dependent acetylation of several histone H3 lysines and preferentially acetylates H3K9 and H3K27 within canonical histone (H3-H4) 2 tetramers. Vps75 shows two protein conformations assembled into dimeric and tetrameric forms, but the roles played by multimeric forms of Vps75 in Rtt109-mediated histone acetylation remain elusive. In P. carinii , we identified that Vps75 (PcVps75) dimers regulate H3K9 and H3K27 acetylation by directly interacting with histone (H3-H4) 2 tetramers, rather than by forming a Vps75-Rtt109 complex. For PcVps75 tetramers, the major histone-binding surface is buried within a walnut-like structure in the absence of a histone cargo. Based on crystal structures of dimeric and tetrameric forms of PcVps75, as well as HAT assay data, we confirmed that residues 192E, 193D, 194E, 195E, and 196E and the disordered C-terminal tail (residues 224-250) of PcVps75 mediate interactions with histones and are important for the Rtt109 in P. carinii (PcRtt109)-mediated acetylation of H3K9 and H3K27, both in vitro and in yeast cells. Furthermore, expressing PcRtt109 alone or in combination with PcVps75 variants that cannot effectively bind histones could not fully restore cellular growth in the presence of genotoxic agents that block DNA replication owing to the absence of H3K9 and H3K27 acetylation. Together, these data indicate that the interaction between PcVps75 and histone (H3-H4) 2 tetramers is a critical regulator of the Rtt109-mediated acetylation of H3K9 and H3K27.
Organizational Affiliation:
1State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, Sichuan P. R. China.