Identification and Experimental Characterization of an Extremophilic Brine Pool Alcohol Dehydrogenase from Single Amplified Genomes
Groetzinger, S.W., Karan, R., Strillinger, E., Bader, S., Frank, A., Al Rowaihi, I.S., Akal, A., Wackerow, W., Archer, J.A., Rueping, M., Weuster-Botz, D., Groll, M., Eppinger, J., Arold, S.T.(2018) ACS Chem Biol 13: 161-170
- PubMed: 29188989
- DOI: https://doi.org/10.1021/acschembio.7b00792
- Primary Citation of Related Structures:
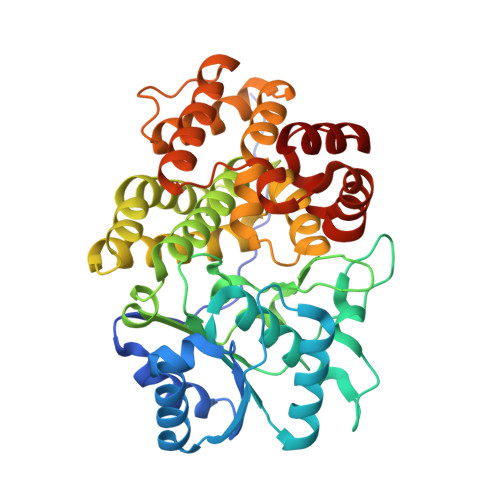
5YVM, 5YVR, 5YVS - PubMed Abstract:
Because only 0.01% of prokaryotic genospecies can be cultured and in situ observations are often impracticable, culture-independent methods are required to understand microbial life and harness potential applications of microbes. Here, we report a methodology for the production of proteins with desired functions based on single amplified genomes (SAGs) from unculturable species. We use this method to resurrect an alcohol dehydrogenase (ADH/D1) from an uncharacterized halo-thermophilic archaeon collected from a brine pool at the bottom of the Red Sea. Our crystal structure of 5,6-dihydroxy NADPH-bound ADH/D1 combined with biochemical analyses reveal the molecular features of its halo-thermophily, its unique habitat adaptations, and its possible reaction mechanism for atypical oxygen activation. Our strategy offers a general guide for using SAGs as a source for scientific and industrial investigations of "microbial dark matter."
Organizational Affiliation:
King Abdullah University of Science and Technology , Biological and Environmental Science and Engineering Division, Computational Bioscience Research Center, Thuwal, Kingdom of Saudi Arabia.