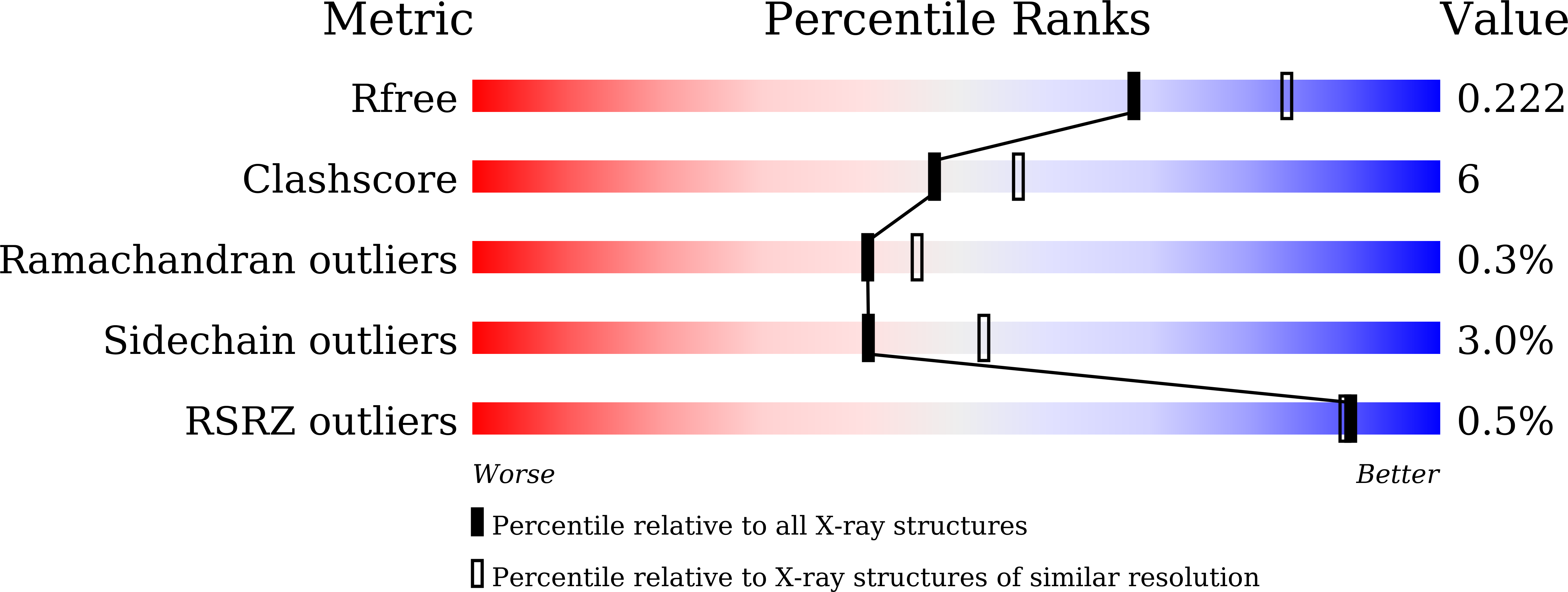
Structural and biochemical characterization of the catalytic domains of GdpP reveals a unified hydrolysis mechanism for the DHH/DHHA1 phosphodiesterase
Wang, F., He, Q., Su, K., Wei, T., Xu, S., Gu, L.(2018) Biochem J 475: 191-205
- PubMed: 29203646
- DOI: https://doi.org/10.1042/BCJ20170739
- Primary Citation of Related Structures:
5XSI, 5XSN, 5XSP, 5XT3 - PubMed Abstract:
The Asp-His-His and Asp-His-His-associated (DHH/DHHA1) domain-containing phosphodiesterases (PDEs) that catalyze degradation of cyclic di-adenosine monophosphate (c-di-AMP) could be subdivided into two subfamilies based on the final product [5'-phosphadenylyl-adenosine (5'-pApA) or AMP]. In a previous study, we revealed that Rv2837c, a stand-alone DHH/DHHA1 PDE, employs a 5'-pApA internal flipping mechanism to produce AMPs. However, why the membrane-bound DHH/DHHA1 PDE can only degrade c-di-AMP to 5'-pApA remains obscure. Here, we report the crystal structure of the DHH/DHHA1 domain of GdpP (GdpP-C), and structures in complex with c-di-AMP, cyclic di-guanosine monophosphate (c-di-GMP), and 5'-pApA. Structural analysis reveals that GdpP-C binds nucleotide substrates quite differently from how Rv2837c does in terms of substrate-binding position. Accordingly, the nucleotide-binding site of the DHH/DHHA1 PDEs is organized into three (C, G, and R) subsites. For GdpP-C, in the C and G sites c-di-AMP binds and degrades into 5'-pApA, and its G site determines nucleotide specificity. To further degrade into AMPs, 5'-pApA must slide into the C and R sites for flipping and hydrolysis as in Rv2837c. Subsequent mutagenesis and enzymatic studies of GdpP-C and Rv2837c uncover the complete flipping process and reveal a unified catalytic mechanism for members of both DHH/DHHA1 PDE subfamilies.
Organizational Affiliation:
State Key Laboratory of Microbial Technology, School of Life Sciences, Shandong University, Jinan, Shandong 250100, China.