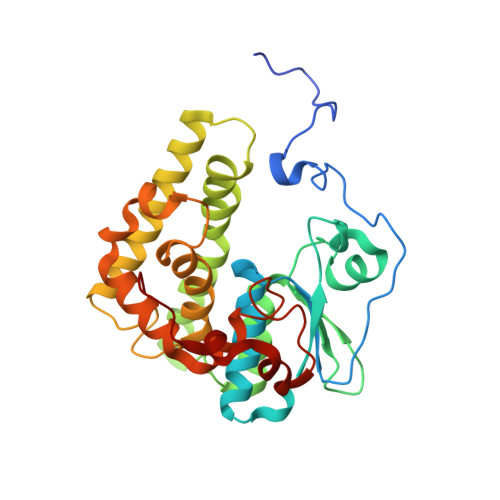
Novosphingobium aromaticivoransuses a Nu-class glutathioneS-transferase as a glutathione lyase in breaking the beta-aryl ether bond of lignin.
Kontur, W.S., Bingman, C.A., Olmsted, C.N., Wassarman, D.R., Ulbrich, A., Gall, D.L., Smith, R.W., Yusko, L.M., Fox, B.G., Noguera, D.R., Coon, J.J., Donohue, T.J.(2018) J Biol Chem 293: 4955-4968
- PubMed: 29449375
- DOI: https://doi.org/10.1074/jbc.RA117.001268
- Primary Citation of Related Structures:
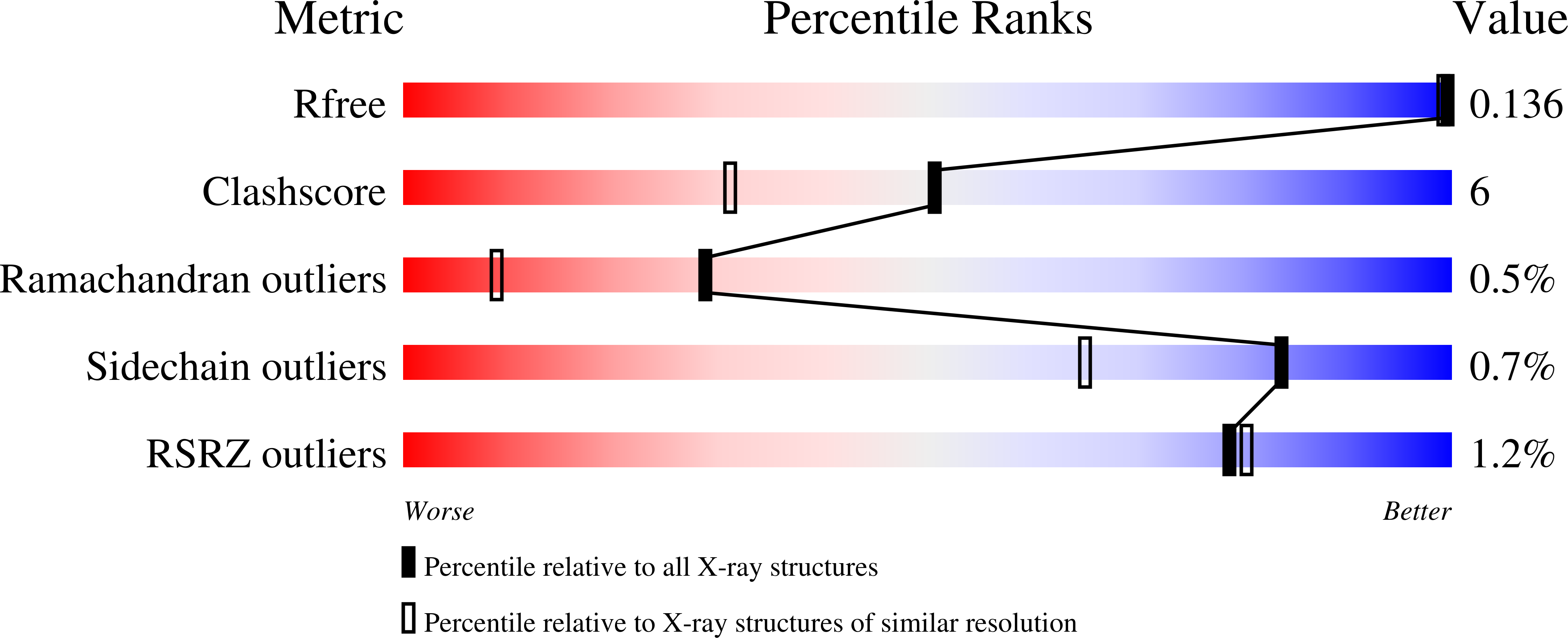
5UUN, 5UUO - PubMed Abstract:
As a major component of plant cell walls, lignin is a potential renewable source of valuable chemicals. Several sphingomonad bacteria have been identified that can break the β-aryl ether bond connecting most phenylpropanoid units of the lignin heteropolymer. Here, we tested three sphingomonads predicted to be capable of breaking the β-aryl ether bond of the dimeric aromatic compound guaiacylglycerol-β-guaiacyl ether (GGE) and found that Novosphingobium aromaticivorans metabolizes GGE at one of the fastest rates thus far reported. After the ether bond of racemic GGE is broken by replacement with a thioether bond involving glutathione, the glutathione moiety must be removed from the resulting two stereoisomers of the phenylpropanoid conjugate β-glutathionyl-γ-hydroxypropiovanillone (GS-HPV). We found that the Nu-class glutathione S -transferase NaGST Nu is the only enzyme needed to remove glutathione from both ( R )- and ( S )-GS-HPV in N. aromaticivorans We solved the crystal structure of NaGST Nu and used molecular modeling to propose a mechanism for the glutathione lyase (deglutathionylation) reaction in which an enzyme-stabilized glutathione thiolate attacks the thioether bond of GS-HPV, and the reaction proceeds through an enzyme-stabilized enolate intermediate. Three residues implicated in the proposed mechanism (Thr 51 , Tyr 166 , and Tyr 224 ) were found to be critical for the lyase reaction. We also found that Nu-class GSTs from Sphingobium sp. SYK-6 (which can also break the β-aryl ether bond) and Escherichia coli (which cannot break the β-aryl ether bond) can also cleave ( R )- and ( S )-GS-HPV, suggesting that glutathione lyase activity may be common throughout this widespread but largely uncharacterized class of glutathione S -transferases.
Organizational Affiliation:
From the Wisconsin Energy Institute.