5' End Nicotinamide Adenine Dinucleotide Cap in Human Cells Promotes RNA Decay through DXO-Mediated deNADding.
Jiao, X., Doamekpor, S.K., Bird, J.G., Nickels, B.E., Tong, L., Hart, R.P., Kiledjian, M.(2017) Cell 168: 1015-1027.e10
- PubMed: 28283058
- DOI: https://doi.org/10.1016/j.cell.2017.02.019
- Primary Citation of Related Structures:
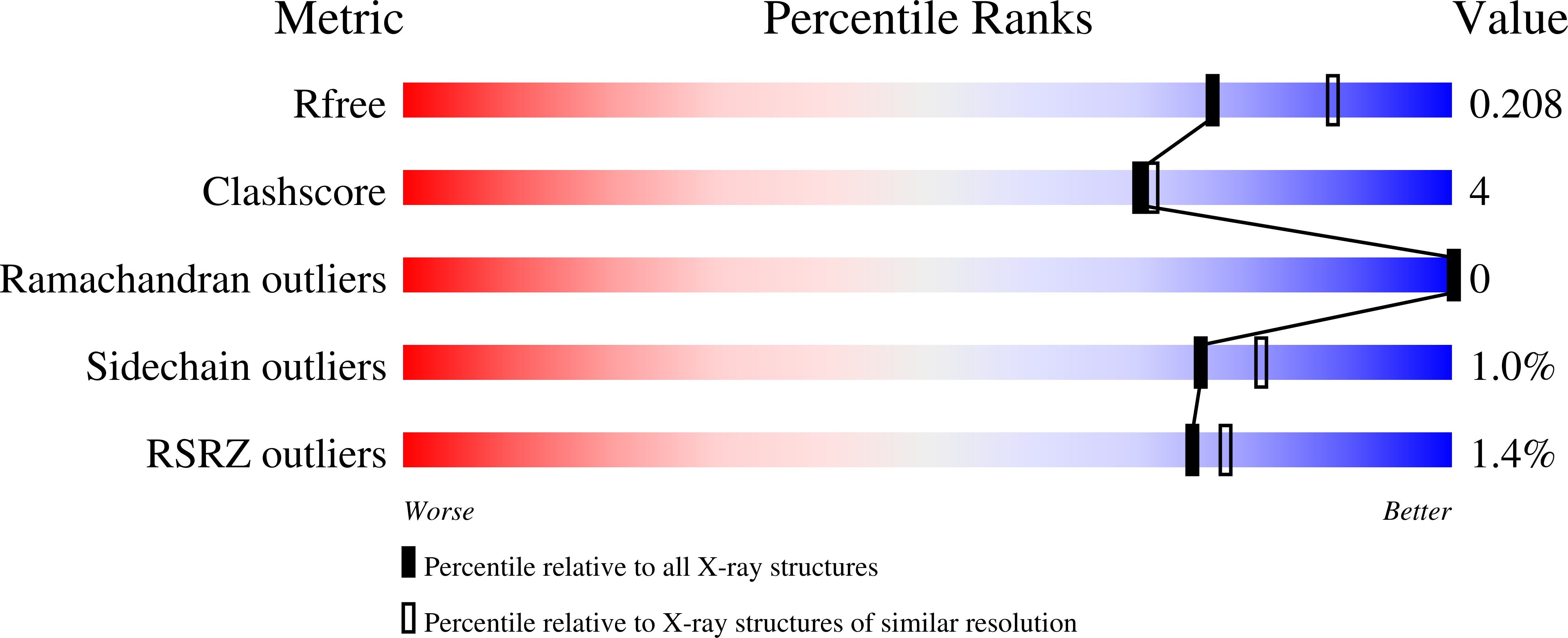
5ULI, 5ULJ - PubMed Abstract:
Eukaryotic mRNAs generally possess a 5' end N7 methyl guanosine (m 7 G) cap that promotes their translation and stability. However, mammalian mRNAs can also carry a 5' end nicotinamide adenine dinucleotide (NAD + ) cap that, in contrast to the m 7 G cap, does not support translation but instead promotes mRNA decay. The mammalian and fungal noncanonical DXO/Rai1 decapping enzymes efficiently remove NAD + caps, and cocrystal structures of DXO/Rai1 with 3'-NADP + illuminate the molecular mechanism for how the "deNADding" reaction produces NAD + and 5' phosphate RNA. Removal of DXO from cells increases NAD + -capped mRNA levels and enables detection of NAD + -capped intronic small nucleolar RNAs (snoRNAs), suggesting NAD + caps can be added to 5'-processed termini. Our findings establish NAD + as an alternative mammalian RNA cap and DXO as a deNADding enzyme modulating cellular levels of NAD + -capped RNAs. Collectively, these data reveal that mammalian RNAs can harbor a 5' end modification distinct from the classical m 7 G cap that promotes rather than inhibits RNA decay.
Organizational Affiliation:
Department of Cell Biology and Neuroscience, Rutgers University, Piscataway, NJ 08854, USA.