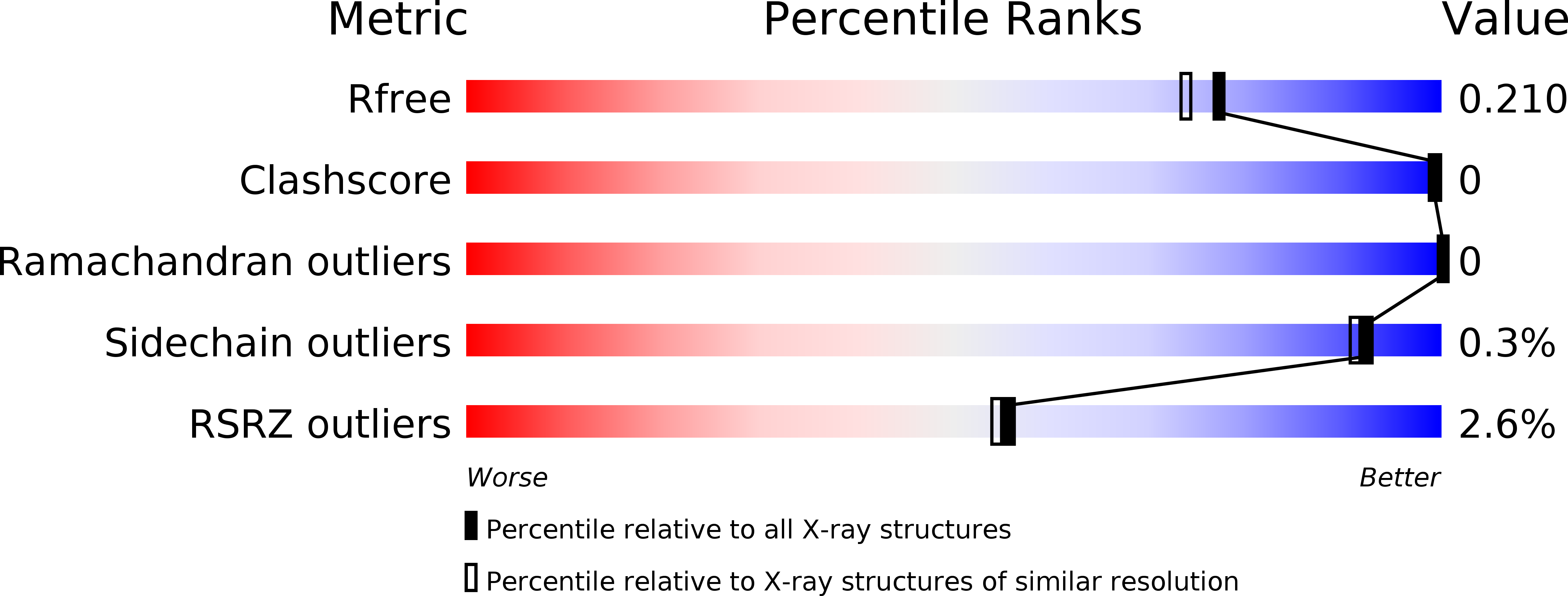
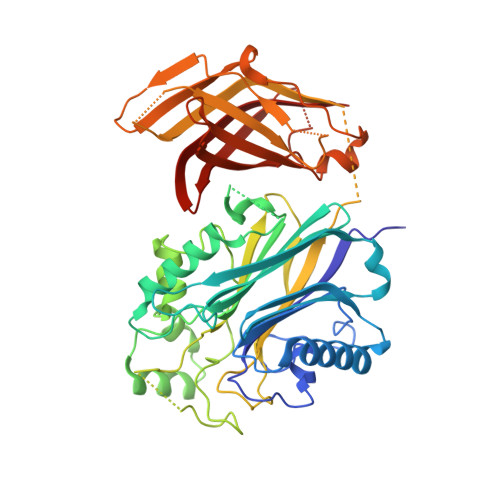
Structural basis for interdomain communication in SHIP2 providing high phosphatase activity.
Le Coq, J., Camacho-Artacho, M., Velazquez, J.V., Santiveri, C.M., Gallego, L.H., Campos-Olivas, R., Dolker, N., Lietha, D.(2017) Elife 6
- PubMed: 28792888
- DOI: https://doi.org/10.7554/eLife.26640
- Primary Citation of Related Structures:
5OKM, 5OKN, 5OKO, 5OKP - PubMed Abstract:
SH2-containing-inositol-5-phosphatases (SHIPs) dephosphorylate the 5-phosphate of phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P 3 ) and play important roles in regulating the PI3K/Akt pathway in physiology and disease. Aiming to uncover interdomain regulatory mechanisms in SHIP2, we determined crystal structures containing the 5-phosphatase and a proximal region adopting a C2 fold. This reveals an extensive interface between the two domains, which results in significant structural changes in the phosphatase domain. Both the phosphatase and C2 domains bind phosphatidylserine lipids, which likely helps to position the active site towards its substrate. Although located distant to the active site, the C2 domain greatly enhances catalytic turnover. Employing molecular dynamics, mutagenesis and cell biology, we identify two distinct allosteric signaling pathways, emanating from hydrophobic or polar interdomain interactions, differentially affecting lipid chain or headgroup moieties of PI(3,4,5)P 3 . Together, this study reveals details of multilayered C2-mediated effects important for SHIP2 activity and points towards interesting new possibilities for therapeutic interventions.
Organizational Affiliation:
Cell Signalling and Adhesion Group, Spanish National Cancer Research Centre, Madrid, Spain.