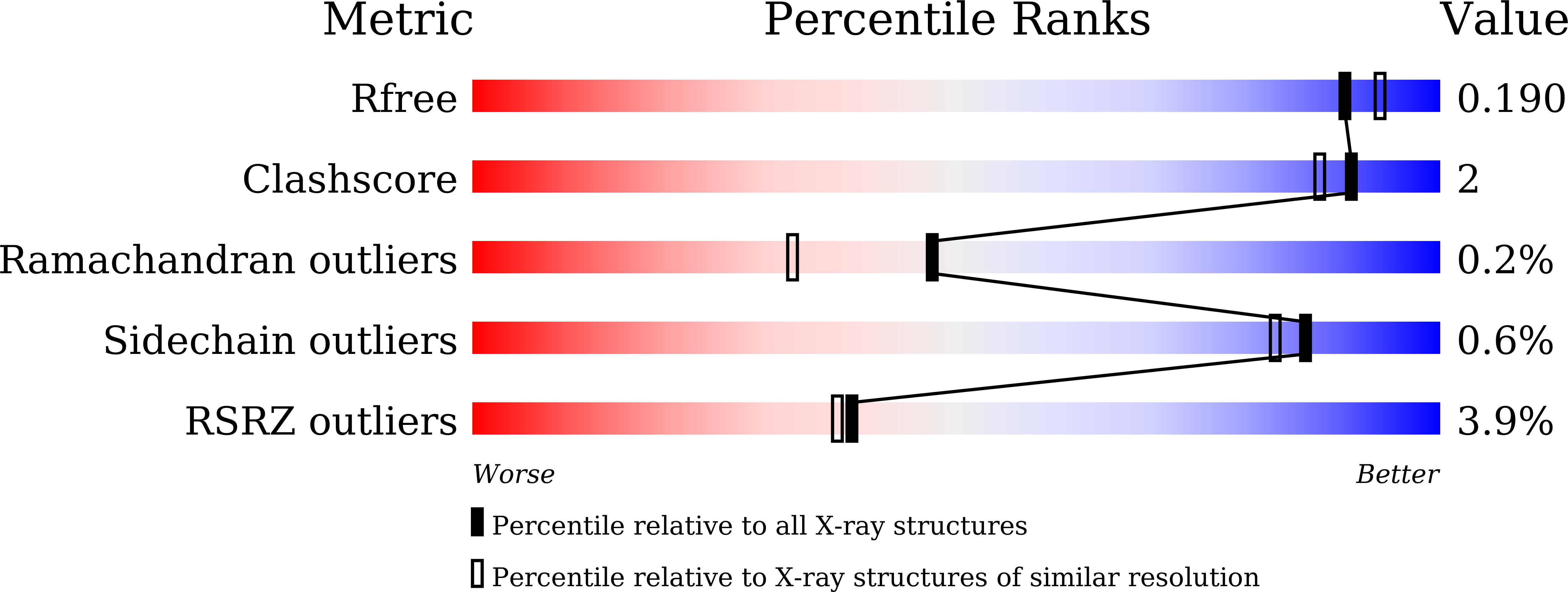
Crystal Structures of Arabidopsis thaliana Oxalyl-CoA Synthetase Essential for Oxalate Degradation
Fan, M., Xiao, Y., Li, M., Chang, W.(2016) Mol Plant 9: 1349-1352
- PubMed: 27326693
- DOI: https://doi.org/10.1016/j.molp.2016.06.002
- Primary Citation of Related Structures:
5IE0, 5IE2, 5IE3
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15th Datun Road, Chaoyang District, Beijing 100101, People's Republic of China; University of Chinese Academy of Sciences, 19A Yuquan Road, Beijing, 100049, People's Republic of China.