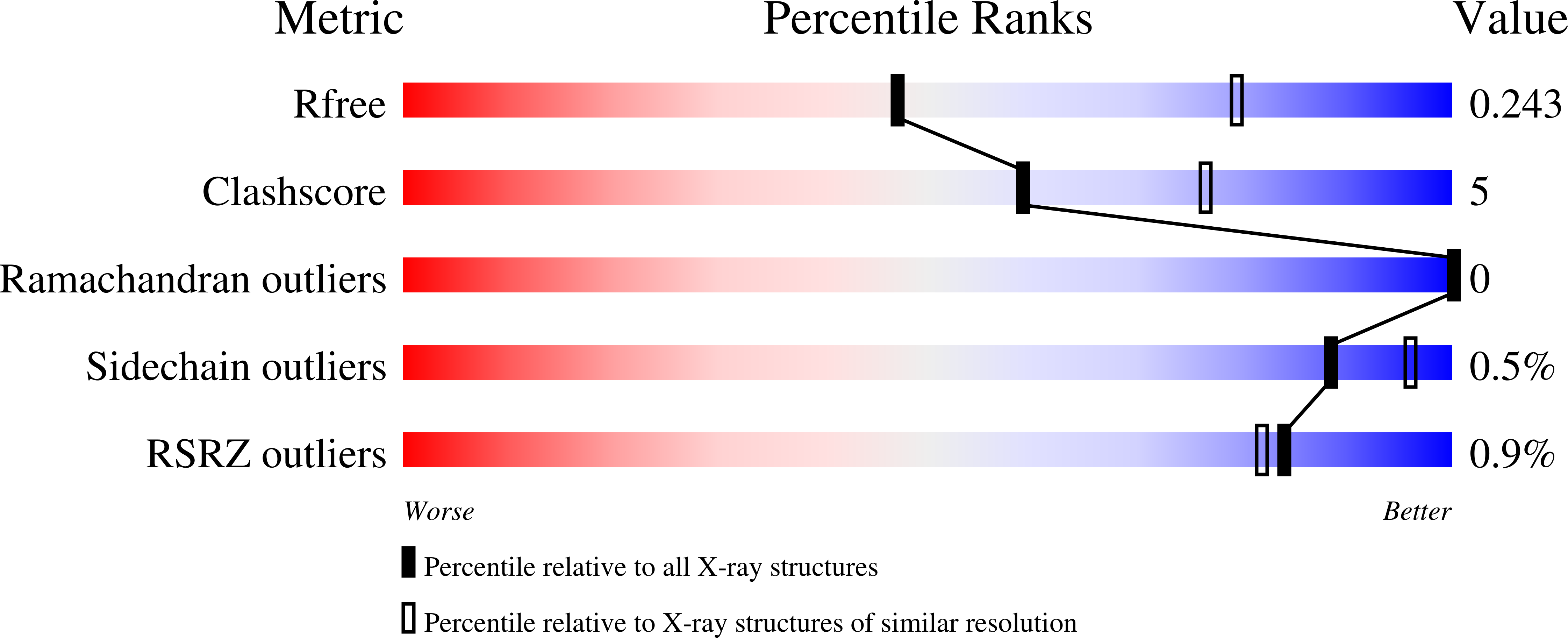
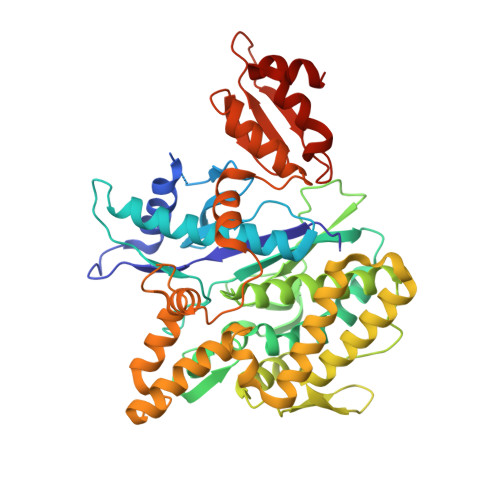
Structures of Pup ligase PafA and depupylase Dop from the prokaryotic ubiquitin-like modification pathway.
Ozcelik, D., Barandun, J., Schmitz, N., Sutter, M., Guth, E., Damberger, F.F., Allain, F.H., Ban, N., Weber-Ban, E.(2012) Nat Commun 3: 1014-1014
- PubMed: 22910360
- DOI: https://doi.org/10.1038/ncomms2009
- Primary Citation of Related Structures:
4B0R, 4B0S, 4B0T - PubMed Abstract:
Pupylation is a posttranslational protein modification occurring in mycobacteria and other actinobacteria that is functionally analogous to ubiquitination. Here we report the crystal structures of the modification enzymes involved in this pathway, the prokaryotic ubiquitin-like protein (Pup) ligase PafA and the depupylase/deamidase Dop. Both feature a larger amino-terminal domain consisting of a central β-sheet packed against a cluster of helices, a fold characteristic for carboxylate-amine ligases, and a smaller C-terminal domain unique to PafA/Dop members. The active site is located on the concave surface of the β-sheet with the nucleotide bound in a deep pocket. A conserved groove leading into the active site could have a role in Pup-binding. Nuclear magnetic resonance and biochemical experiments determine the region of Pup that interacts with PafA and Dop. Structural data and mutational studies identify crucial residues for the catalysis of both enzymes.
Organizational Affiliation:
ETH Zurich, Institute of Molecular Biology & Biophysics, CH-8093, Switzerland.