N-terminal acetylation acts as an avidity enhancer within an interconnected multiprotein complex.
Scott, D.C., Monda, J.K., Bennett, E.J., Harper, J.W., Schulman, B.A.(2011) Science 334: 674-678
- PubMed: 21940857
- DOI: https://doi.org/10.1126/science.1209307
- Primary Citation of Related Structures:
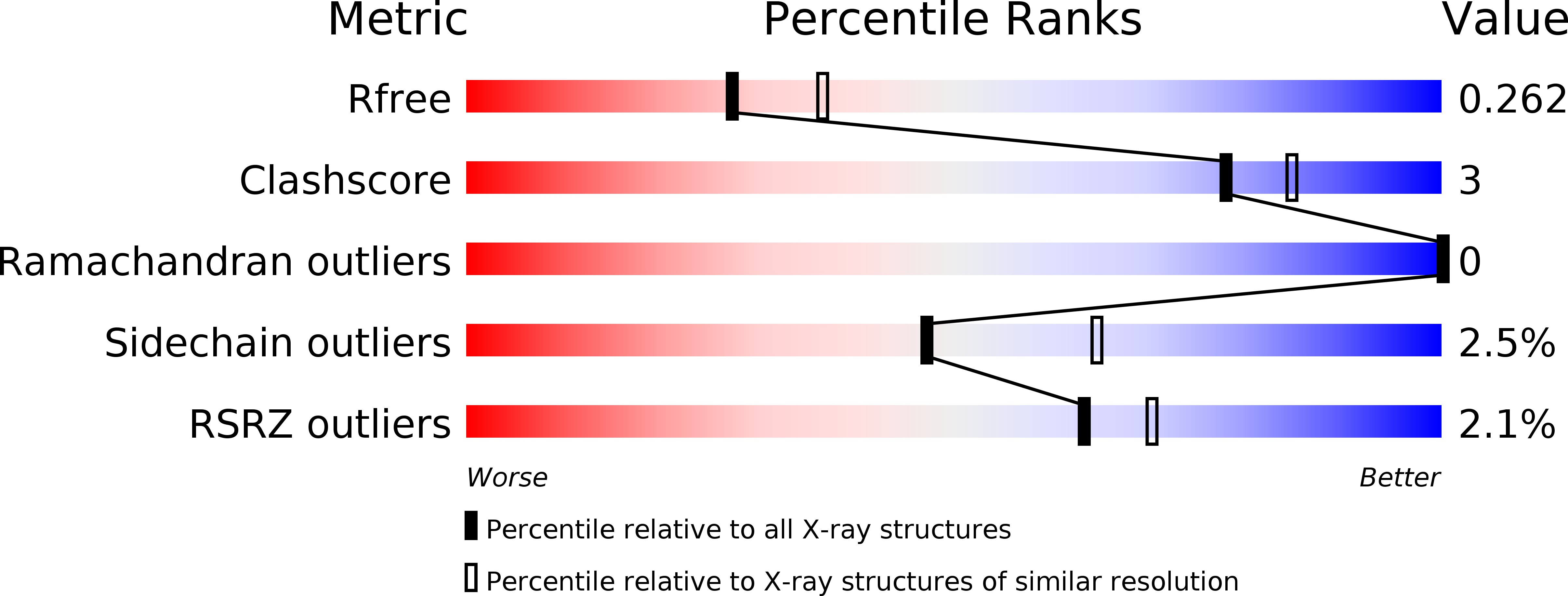
3TDI, 3TDU, 3TDZ - PubMed Abstract:
Although many eukaryotic proteins are amino (N)-terminally acetylated, structural mechanisms by which N-terminal acetylation mediates protein interactions are largely unknown. Here, we found that N-terminal acetylation of the E2 enzyme, Ubc12, dictates distinctive E3-dependent ligation of the ubiquitin-like protein Nedd8 to Cul1. Structural, biochemical, biophysical, and genetic analyses revealed how complete burial of Ubc12's N-acetyl-methionine in a hydrophobic pocket in the E3, Dcn1, promotes cullin neddylation. The results suggest that the N-terminal acetyl both directs Ubc12's interactions with Dcn1 and prevents repulsion of a charged N terminus. Our data provide a link between acetylation and ubiquitin-like protein conjugation and define a mechanism for N-terminal acetylation-dependent recognition.
Organizational Affiliation:
Structural Biology Department, St. Jude Children's Research Hospital, Memphis, TN 38105, USA.