A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors.
Pyburn, T.M., Bensing, B.A., Xiong, Y.Q., Melancon, B.J., Tomasiak, T.M., Ward, N.J., Yankovskaya, V., Oliver, K.M., Cecchini, G., Sulikowski, G.A., Tyska, M.J., Sullam, P.M., Iverson, T.M.(2011) PLoS Pathog 7: e1002112-e1002112
- PubMed: 21765814
- DOI: https://doi.org/10.1371/journal.ppat.1002112
- Primary Citation of Related Structures:
3QC5, 3QC6, 5IUC - PubMed Abstract:
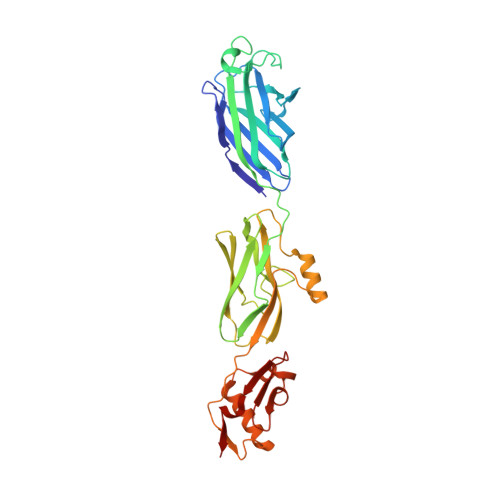
GspB is a serine-rich repeat (SRR) adhesin of Streptococcus gordonii that mediates binding of this organism to human platelets via its interaction with sialyl-T antigen on the receptor GPIbα. This interaction appears to be a major virulence determinant in the pathogenesis of infective endocarditis. To address the mechanism by which GspB recognizes its carbohydrate ligand, we determined the high-resolution x-ray crystal structure of the GspB binding region (GspB(BR)), both alone and in complex with a disaccharide precursor to sialyl-T antigen. Analysis of the GspB(BR) structure revealed that it is comprised of three independently folded subdomains or modules: 1) an Ig-fold resembling a CnaA domain from prokaryotic pathogens; 2) a second Ig-fold resembling the binding region of mammalian Siglecs; 3) a subdomain of unique fold. The disaccharide was found to bind in a pocket within the Siglec subdomain, but at a site distinct from that observed in mammalian Siglecs. Confirming the biological relevance of this binding pocket, we produced three isogenic variants of S. gordonii, each containing a single point mutation of a residue lining this binding pocket. These variants have reduced binding to carbohydrates of GPIbα. Further examination of purified GspB(BR)-R484E showed reduced binding to sialyl-T antigen while S. gordonii harboring this mutation did not efficiently bind platelets and showed a significant reduction in virulence, as measured by an animal model of endocarditis. Analysis of other SRR proteins revealed that the predicted binding regions of these adhesins also had a modular organization, with those known to bind carbohydrate receptors having modules homologous to the Siglec and Unique subdomains of GspB(BR). This suggests that the binding specificity of the SRR family of adhesins is determined by the type and organization of discrete modules within the binding domains, which may affect the tropism of organisms for different tissues.
Organizational Affiliation:
Department of Pharmacology, Vanderbilt University Medical Center, Nashville, Tennessee, United States of America.