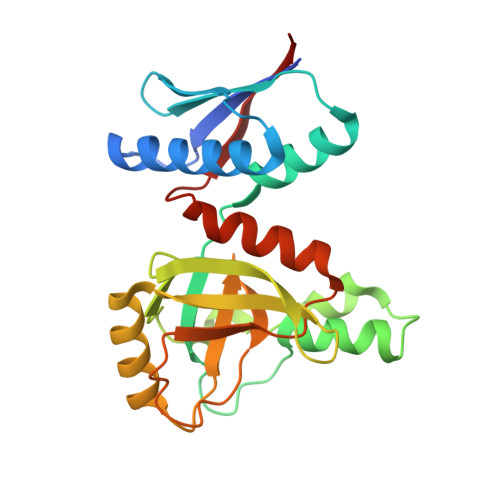
Structure and function of a membrane component SecDF that enhances protein export.
Tsukazaki, T., Mori, H., Echizen, Y., Ishitani, R., Fukai, S., Tanaka, T., Perederina, A., Vassylyev, D.G., Kohno, T., Maturana, A.D., Ito, K., Nureki, O.(2011) Nature 474: 235-238
- PubMed: 21562494
- DOI: https://doi.org/10.1038/nature09980
- Primary Citation of Related Structures:
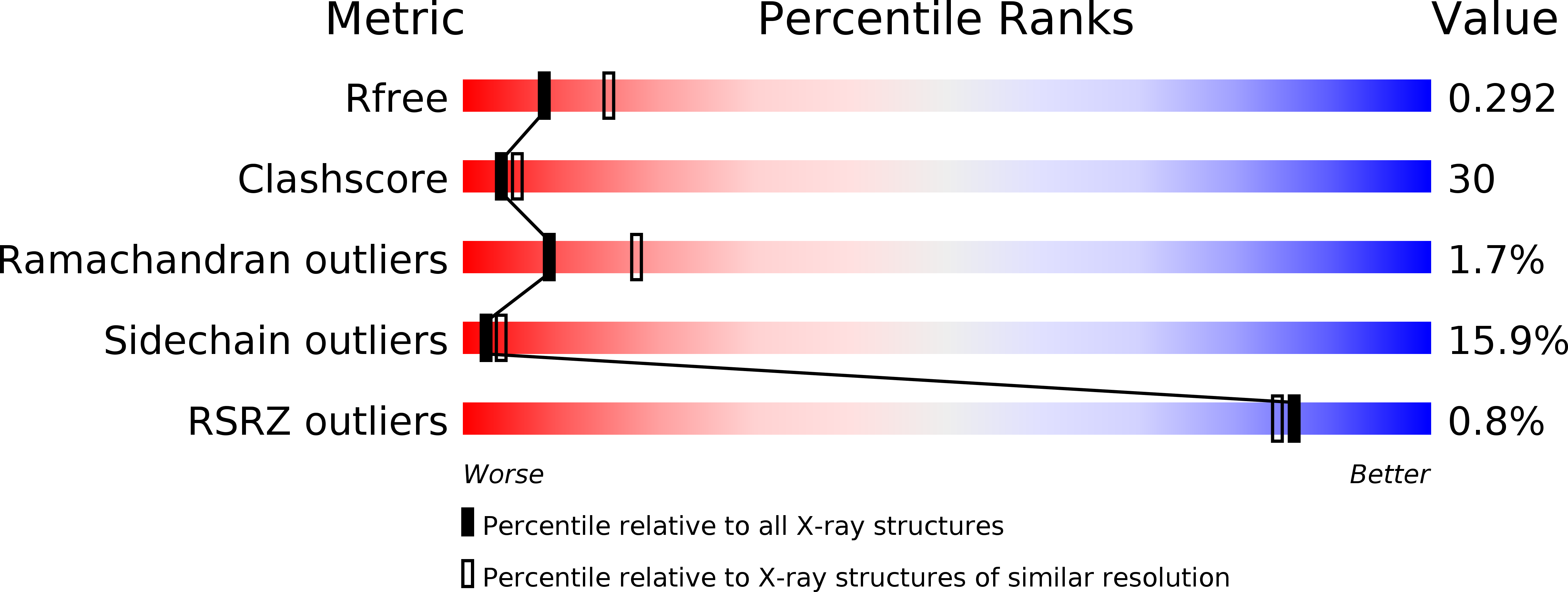
2RRN, 3AQO, 3AQP - PubMed Abstract:
Protein translocation across the bacterial membrane, mediated by the secretory translocon SecYEG and the SecA ATPase, is enhanced by proton motive force and membrane-integrated SecDF, which associates with SecYEG. The role of SecDF has remained unclear, although it is proposed to function in later stages of translocation as well as in membrane protein biogenesis. Here, we determined the crystal structure of Thermus thermophilus SecDF at 3.3 Å resolution, revealing a pseudo-symmetrical, 12-helix transmembrane domain belonging to the RND superfamily and two major periplasmic domains, P1 and P4. Higher-resolution analysis of the periplasmic domains suggested that P1, which binds an unfolded protein, undergoes functionally important conformational changes. In vitro analyses identified an ATP-independent step of protein translocation that requires both SecDF and proton motive force. Electrophysiological analyses revealed that SecDF conducts protons in a manner dependent on pH and the presence of an unfolded protein, with conserved Asp and Arg residues at the transmembrane interface between SecD and SecF playing essential roles in the movements of protons and preproteins. Therefore, we propose that SecDF functions as a membrane-integrated chaperone, powered by proton motive force, to achieve ATP-independent protein translocation.
Organizational Affiliation:
Department of Biophysics and Biochemistry, Graduate School of Science, The University of Tokyo, Bunkyo-ku, Tokyo 113-0032, Japan.