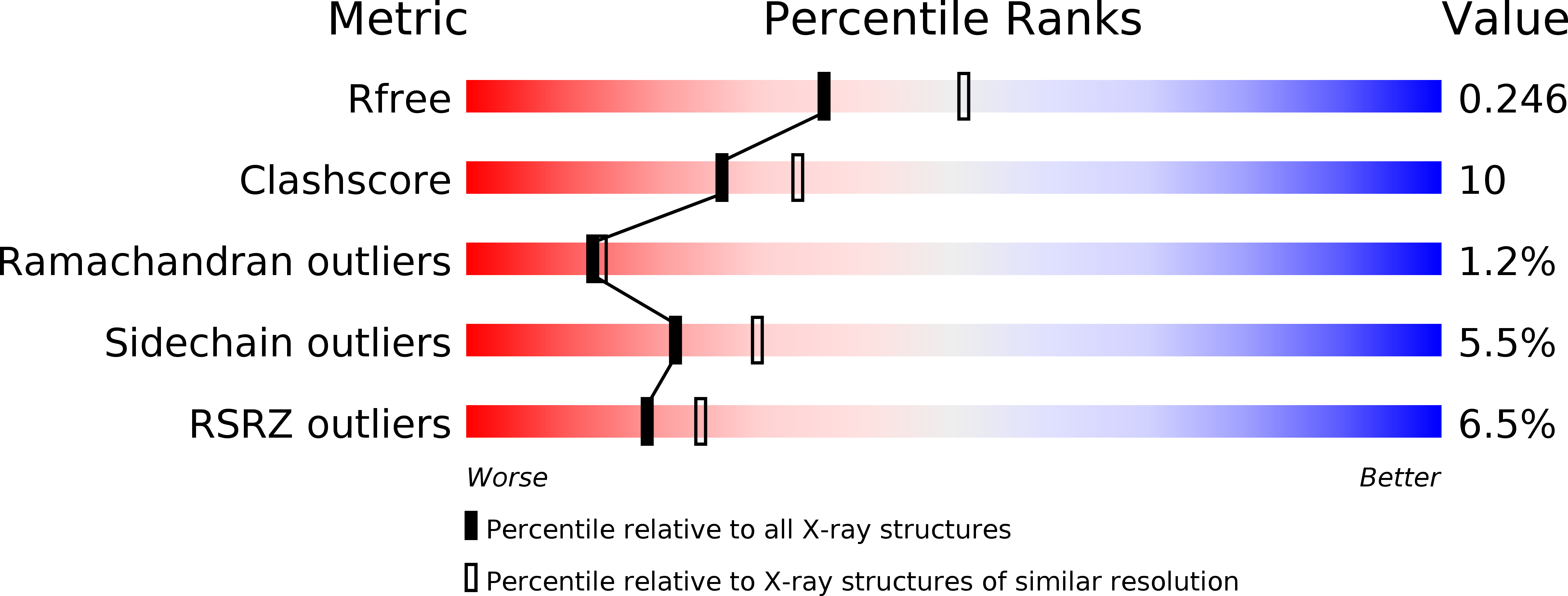
Bifunctional NMN Adenylyltransferase/ADP-Ribose Pyrophosphatase: Structure and Function in Bacterial NAD Metabolism.
Huang, N., Sorci, L., Zhang, X., Brautigam, C.A., Li, X., Raffaelli, N., Magni, G., Grishin, N.V., Osterman, A.L., Zhang, H.(2008) Structure 16: 196-209
- PubMed: 18275811
- DOI: https://doi.org/10.1016/j.str.2007.11.017
- Primary Citation of Related Structures:
2QJO, 2QJT, 2R5W - PubMed Abstract:
Bacterial NadM-Nudix is a bifunctional enzyme containing a nicotinamide mononucleotide (NMN) adenylyltransferase and an ADP-ribose (ADPR) pyrophosphatase domain. While most members of this enzyme family, such as that from a model cyanobacterium Synechocystis sp., are involved primarily in nicotinamide adenine dinucleotide (NAD) salvage/recycling pathways, its close homolog in a category-A biodefense pathogen, Francisella tularensis, likely plays a central role in a recently discovered novel pathway of NAD de novo synthesis. The crystal structures of NadM-Nudix from both species, including their complexes with various ligands and catalytic metal ions, revealed detailed configurations of the substrate binding and catalytic sites in both domains. The structure of the N-terminal NadM domain may be exploited for designing new antitularemia therapeutics. The ADPR binding site in the C-terminal Nudix domain is substantially different from that of Escherichia coli ADPR pyrophosphatase, and is more similar to human NUDT9. The latter observation provided new insights into the ligand binding mode of ADPR-gated Ca2+ channel TRPM2.
Organizational Affiliation:
Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.