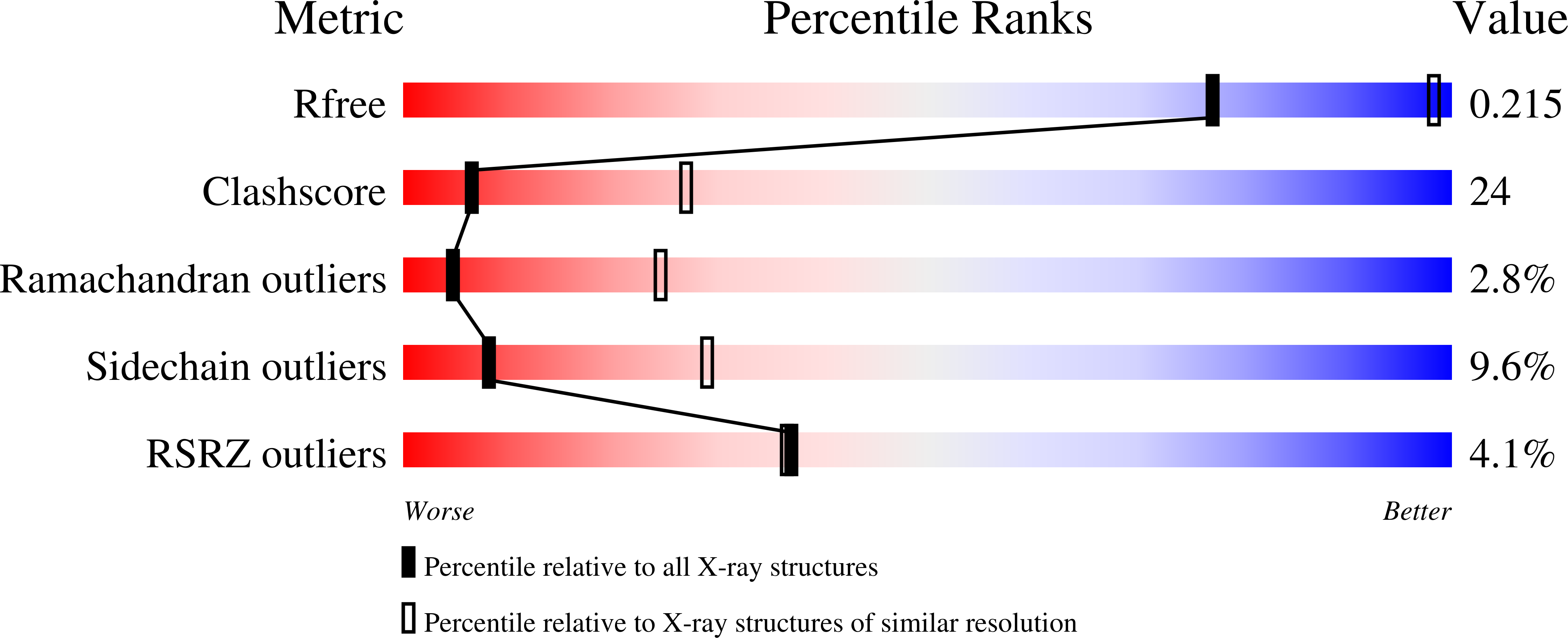
Crystal structures of four types of human papillomavirus L1 capsid proteins: understanding the specificity of neutralizing monoclonal antibodies.
Bishop, B., Dasgupta, J., Klein, M., Garcea, R.L., Christensen, N.D., Zhao, R., Chen, X.S.(2007) J Biol Chem 282: 31803-31811
- PubMed: 17804402
- DOI: https://doi.org/10.1074/jbc.M706380200
- Primary Citation of Related Structures:
2R5H, 2R5I, 2R5J, 2R5K - PubMed Abstract:
Human papillomaviruses (HPVs) are known etiologic agents of cervical cancer. Vaccines that contain virus-like particles (VLPs) made of L1 capsid protein from several high risk HPV types have proven to be effective against HPV infections. Raising high levels of neutralizing antibodies against each HPV type is believed to be the primary mechanism of protection, gained by vaccination. Antibodies elicited by a particular HPV type are highly specific to that particular HPV type and show little or no cross-reactivity between HPV types. With an intention to understand the interplay between the L1 structure of different HPV types and the type specificity of neutralizing antibodies, we have prepared the L1 pentamers of four different HPV types, HPV11, HPV16, HPV18, and HPV35. The pentamers only bind the type-specific neutralizing monoclonal antibodies (NmAbs) that are raised against the VLP of the corresponding HPV type, implying that the surface loop structures of the pentamers from each type are distinctive and functionally active as VLPs in terms of antibody binding. We have determined the crystal structures of all four L1 pentamers, and their comparisons revealed characteristic conformational differences of the surface loops that contain the known epitopes for the NmAbs. On the basis of these distinct surface loop structures, we have provided a molecular explanation for the type specificity of NmAbs against HPV infection.
Organizational Affiliation:
Department of Molecular and Computational Biology, University of Southern California, Los Angeles, California 90089, USA.