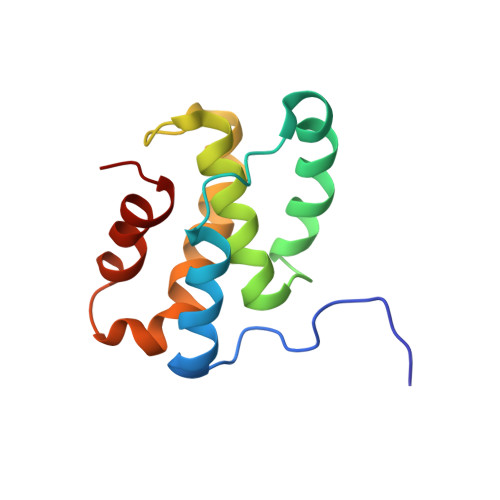
Structural Insights into RNA Polymerase Recognition and Essential Function of Myxococcus xanthus CdnL.
Gallego-Garcia, A., Mirassou, Y., Garcia-Moreno, D., Elias-Arnanz, M., Jimenez, M.A., Padmanabhan, S.(2014) PLoS One 9: e108946-e108946
- PubMed: 25272012
- DOI: https://doi.org/10.1371/journal.pone.0108946
- Primary Citation of Related Structures:
2LT3, 2LT4, 2LWJ - PubMed Abstract:
CdnL and CarD are two functionally distinct members of the CarD_CdnL_TRCF family of bacterial RNA polymerase (RNAP)-interacting proteins, which co-exist in Myxococcus xanthus. While CarD, found exclusively in myxobacteria, has been implicated in the activity of various extracytoplasmic function (ECF) σ-factors, the function and mode of action of the essential CdnL, whose homologs are widespread among bacteria, remain to be elucidated in M. xanthus. Here, we report the NMR solution structure of CdnL and present a structure-based mutational analysis of its function. An N-terminal five-stranded β-sheet Tudor-like module in the two-domain CdnL mediates binding to RNAP-β, and mutations that disrupt this interaction impair cell growth. The compact CdnL C-terminal domain consists of five α-helices folded as in some tetratricopeptide repeat-like protein-protein interaction domains, and contains a patch of solvent-exposed nonpolar and basic residues, among which a set of basic residues is shown to be crucial for CdnL function. We show that CdnL, but not its loss-of-function mutants, stabilizes formation of transcriptionally competent, open complexes by the primary σA-RNAP holoenzyme at an rRNA promoter in vitro. Consistent with this, CdnL is present at rRNA promoters in vivo. Implication of CdnL in RNAP-σA activity and of CarD in ECF-σ function in M. xanthus exemplifies how two related members within a widespread bacterial protein family have evolved to enable distinct σ-dependent promoter activity.
Organizational Affiliation:
Departamento de Genética y Microbiología, Área de Genética (Unidad Asociada al IQFR-CSIC), Facultad de Biología, Universidad de Murcia, Murcia, Spain.