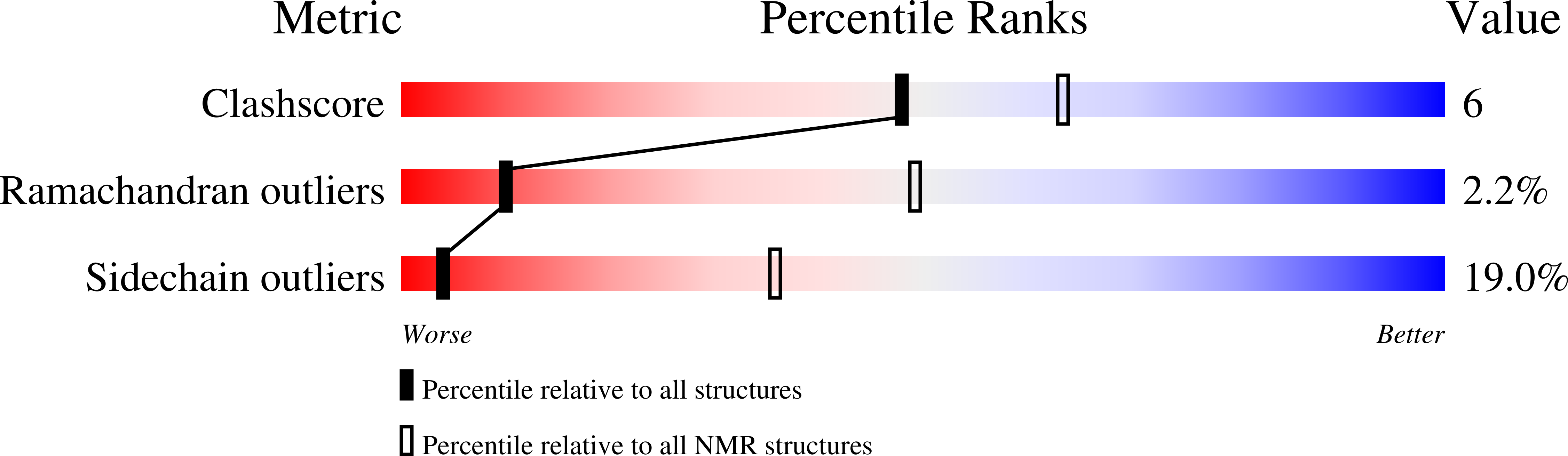Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo.
Wu, L., Pan, L., Wei, Z., Zhang, M.(2011) Science 331: 757-760
- PubMed: 21311020
- DOI: https://doi.org/10.1126/science.1198848
- Primary Citation of Related Structures:
2L7T, 3PVL - PubMed Abstract:
The unconventional myosin VIIa (MYO7A) is one of the five proteins that form a network of complexes involved in formation of stereocilia. Defects in these proteins cause syndromic deaf-blindness in humans [Usher syndrome I (USH1)]. Many disease-causing mutations occur in myosin tail homology 4-protein 4.1, ezrin, radixin, moesin (MyTH4-FERM) domains in the myosin tail that binds to another USH1 protein, Sans. We report the crystal structure of MYO7A MyTH4-FERM domains in complex with the central domain (CEN) of Sans at 2.8 angstrom resolution. The MyTH4 and FERM domains form an integral structural and functional supramodule binding to two highly conserved segments (CEN1 and 2) of Sans. The MyTH4-FERM/CEN complex structure provides mechanistic explanations for known deafness-causing mutations in MYO7A MyTH4-FERM. The structure will also facilitate mechanistic and functional studies of MyTH4-FERM domains in other myosins.
Organizational Affiliation:
Division of Life Science, Molecular Neuroscience Center, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong.