Crystallographic Snapshots of Oxalyl-Coa Decarboxylase Give Insights Into Catalysis by Nonoxidative Thdp-Dependent Decarboxylases
Berthold, C.L., Toyota, C.G., Moussatche, P., Wood, M.D., Leeper, F., Richards, N.G.J., Lindqvist, Y.(2007) Structure 15: 853
- PubMed: 17637344
- DOI: https://doi.org/10.1016/j.str.2007.06.001
- Primary Citation of Related Structures:
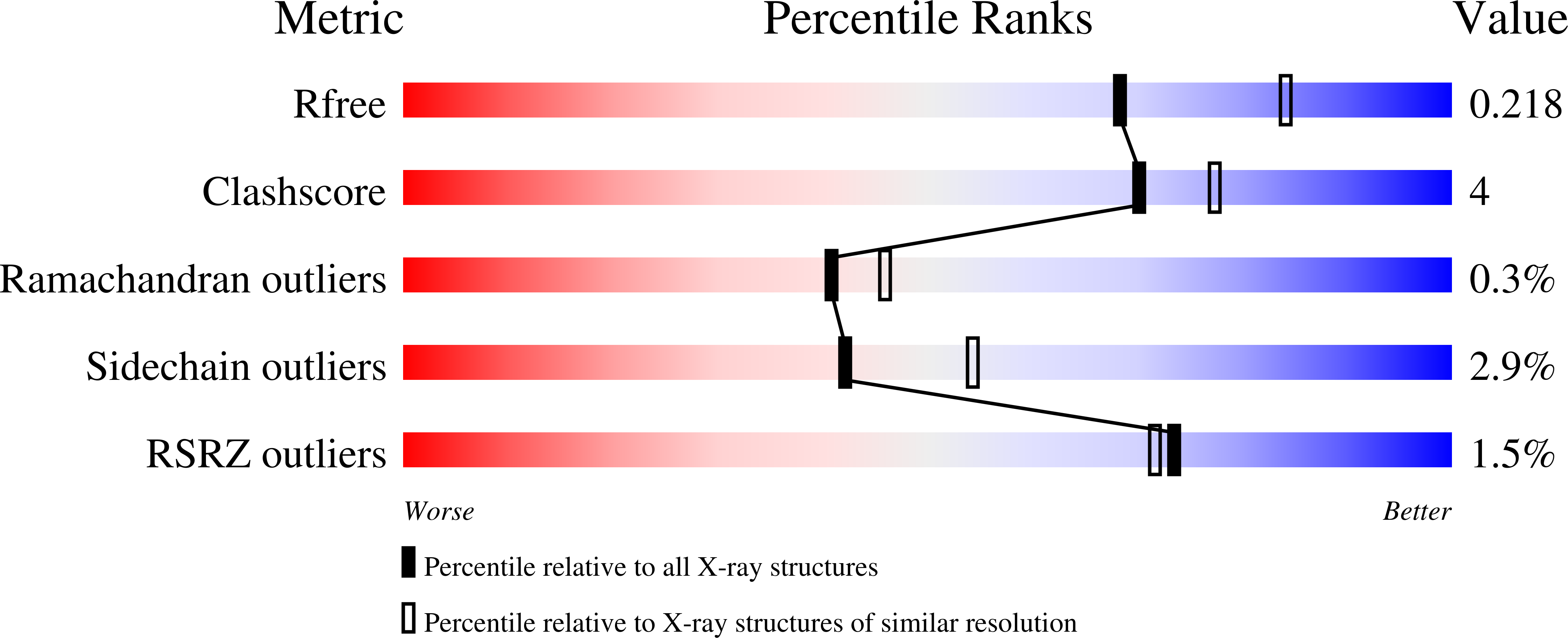
2JI6, 2JI7, 2JI8, 2JI9, 2JIB - PubMed Abstract:
Despite more than five decades of extensive studies of thiamin diphosphate (ThDP) enzymes, there remain many uncertainties as to how these enzymes achieve their rate enhancements. Here, we present a clear picture of catalysis for the simple nonoxidative decarboxylase, oxalyl-coenzyme A (CoA) decarboxylase, based on crystallographic snapshots along the catalytic cycle and kinetic data on active site mutants. First, we provide crystallographic evidence that, upon binding of oxalyl-CoA, the C-terminal 13 residues fold over the substrate, aligning the substrate alpha-carbon for attack by the ThDP-C2 atom. The second structure presented shows a covalent reaction intermediate after decarboxylation, interpreted as being nonplanar. Finally, the structure of a product complex is presented. In accordance with mutagenesis data, no side chains of the enzyme are implied to directly participate in proton transfer except the glutamic acid (Glu-56), which promotes formation of the 1',4'-iminopyrimidine tautomer of ThDP needed for activation.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Molecular Structural Biology, Karolinska Institutet, S-17177 Stockholm, Sweden.