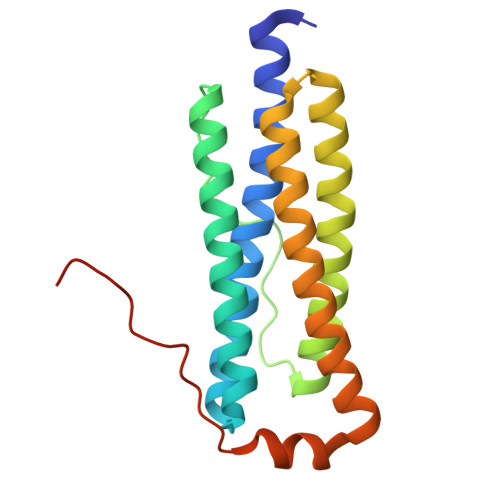
The nature of the di-iron site in the bacterioferritin from Desulfovibrio desulfuricans
Macedo, S., Romao, C.V., Mitchell, E., Matias, P.M., Liu, M.Y., Xavier, A.V., LeGall, J., Teixeira, M., Lindley, P., Carrondo, M.A.(2003) Nat Struct Biol 10: 285-290
- PubMed: 12627224
- DOI: https://doi.org/10.1038/nsb909
- Primary Citation of Related Structures:
1NF4, 1NF6, 1NFV - PubMed Abstract:
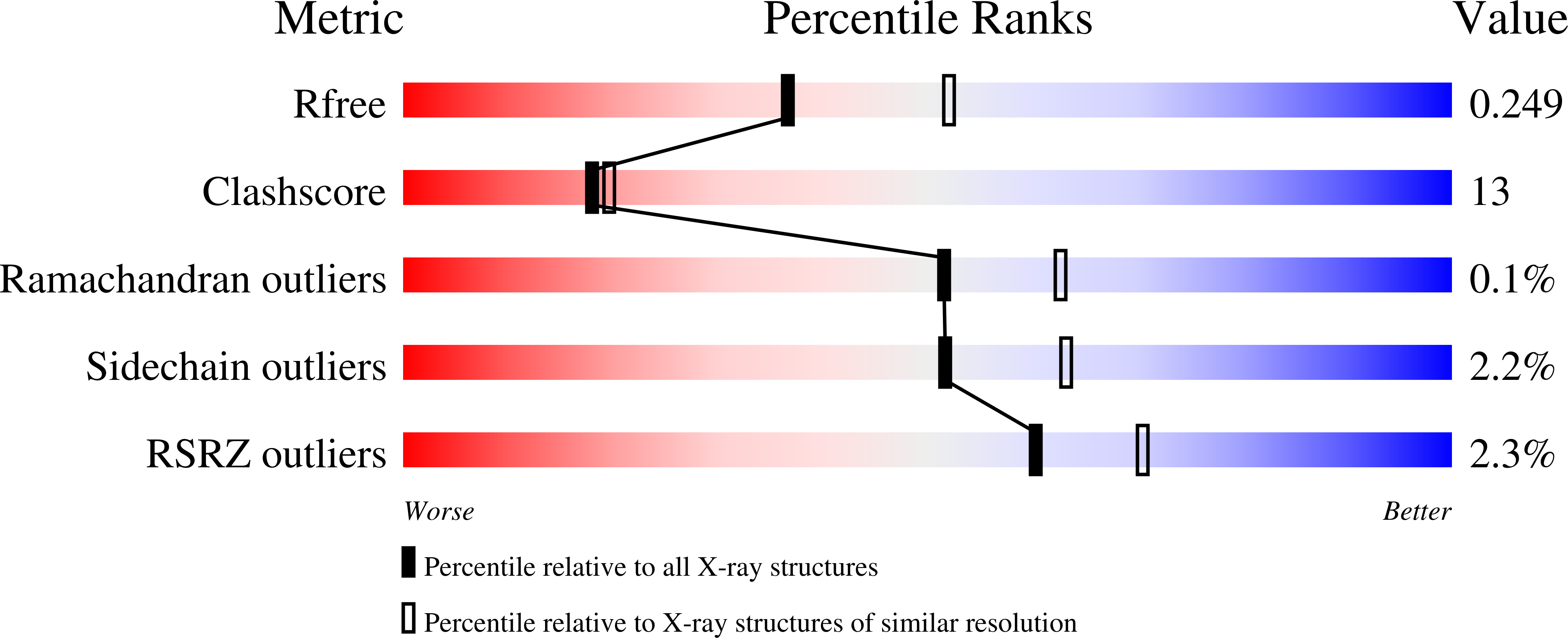
The first crystal structure of a native di-iron center in an iron-storage protein (bacterio)ferritin is reported. The protein, isolated from the anaerobic bacterium Desulfovibrio desulfuricans, has the unique property of having Fe-coproporphyrin III as its heme cofactor. The three-dimensional structure of this bacterioferritin was determined in three distinct catalytic/redox states by X-ray crystallography (at 1.95, 2.05 and 2.35 A resolution), corresponding to different intermediates of the di-iron ferroxidase site. Conformational changes associated with these intermediates support the idea of a route for iron entry into the protein shell through a pore that passes through the di-iron center. Molecular surface and electrostatic potential calculations also suggest the presence of another ion channel, distant from the channels at the three- and four-fold axes proposed as points of entry for the iron atoms.
Organizational Affiliation:
Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Av. República, EAN, Apartado 127, 2781-901 Oeiras, Portugal.