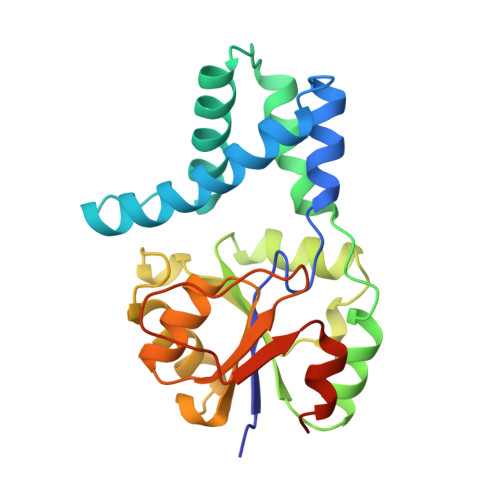
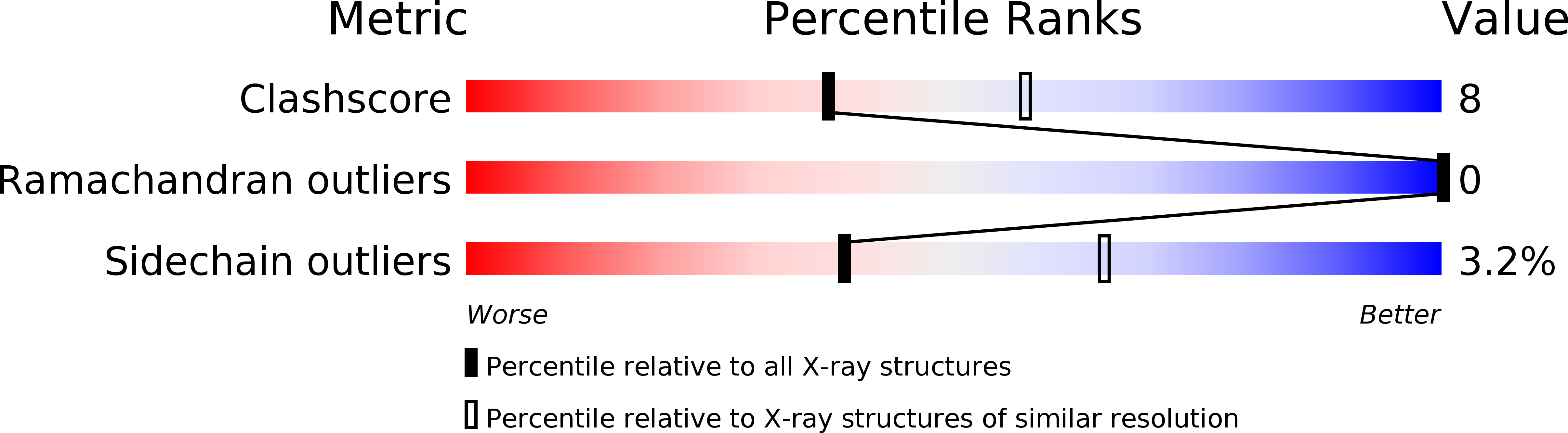Crystal structure of L-2-haloacid dehalogenase from Pseudomonas sp. YL. An alpha/beta hydrolase structure that is different from the alpha/beta hydrolase fold.
Hisano, T., Hata, Y., Fujii, T., Liu, J.Q., Kurihara, T., Esaki, N., Soda, K.(1996) J Biol Chem 271: 20322-20330
- PubMed: 8702766
- DOI: https://doi.org/10.1074/jbc.271.34.20322
- Primary Citation of Related Structures:
1JUD - PubMed Abstract:
L-2-Haloacid dehalogenase catalyzes the hydrolytic dehalogenation of L-2-haloalkanoic acids to yield the corresponding D-2-hydroxyalkanoic acids. The crystal structure of the homodimeric enzyme from Pseudomonas sp. YL has been determined by a multiple isomorphous replacement method and refined at 2.5 A resolution to a crystallographic R-factor of 19.5%. The subunit consists of two structurally distinct domains: the core domain and the subdomain. The core domain has an alpha/beta structure formed by a six-stranded parallel beta-sheet flanked by five alpha-helices. The subdomain inserted into the core domain has a four helix bundle structure providing the greater part of the interface for dimer formation. There is an active site cavity between the domains. An experimentally identified nucleophilic residue, Asp-10, is located on a loop following the amino-terminal beta-strand in the core domain, and other functional residues, Thr-14, Arg-41, Ser-118, Lys-151, Tyr-157, Ser-175, Asn-177, and Asp-180, detected by a site-directed mutagenesis experiment, are arranged around the nucleophile in the active site. Although the enzyme is an alpha/beta-type hydrolase, it does not belong to the alpha/beta hydrolase fold family, from the viewpoint of the topological feature and the position of the nucleophile.
Organizational Affiliation:
Institute for Chemical Research, Kyoto University, Uji, Kyoto 611, Japan.