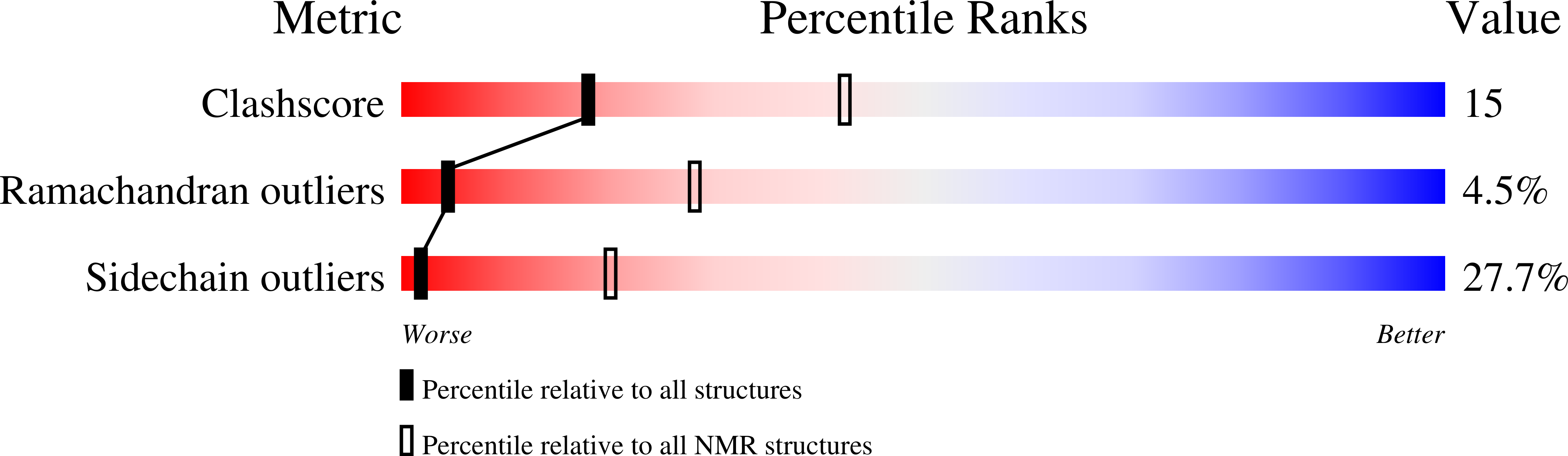The SAND domain structure defines a novel DNA-binding fold in transcriptional regulation.
Bottomley, M.J., Collard, M.W., Huggenvik, J.I., Liu, Z., Gibson, T.J., Sattler, M.(2001) Nat Struct Biol 8: 626-633
- PubMed: 11427895
- DOI: https://doi.org/10.1038/89675
- Primary Citation of Related Structures:
1H5P - PubMed Abstract:
The SAND domain is a conserved sequence motif found in a number of nuclear proteins, including the Sp100 family and NUDR. These are thought to play important roles in chromatin-dependent transcriptional regulation and are linked to many diseases. We have determined the three-dimensional (3D) structure of the SAND domain from Sp100b. The structure represents a novel alpha/beta fold, in which a conserved KDWK sequence motif is found within an alpha-helical, positively charged surface patch. For NUDR, the SAND domain is shown to be sufficient to mediate DNA binding. Using mutational analyses and chemical shift perturbation experiments, the DNA binding surface is mapped to the alpha-helical region encompassing the KDWK motif. The DNA binding activity of wild type and mutant proteins in vitro correlates with transcriptional regulation activity of full length NUDR in vivo. The evolutionarily conserved SAND domain defines a new DNA binding fold that is involved in chromatin-associated transcriptional regulation.
Organizational Affiliation:
European Molecular Biology Laboratory, Meyerhofstrasse 1, 69117 Heidelberg, Germany.