Chloroplast NADP-malate dehydrogenase: structural basis of light-dependent regulation of activity by thiol oxidation and reduction.
Carr, P.D., Verger, D., Ashton, A.R., Ollis, D.L.(1999) Structure 7: 461-475
- PubMed: 10196131
- DOI: https://doi.org/10.1016/s0969-2126(99)80058-6
- Primary Citation of Related Structures:
1CIV - PubMed Abstract:
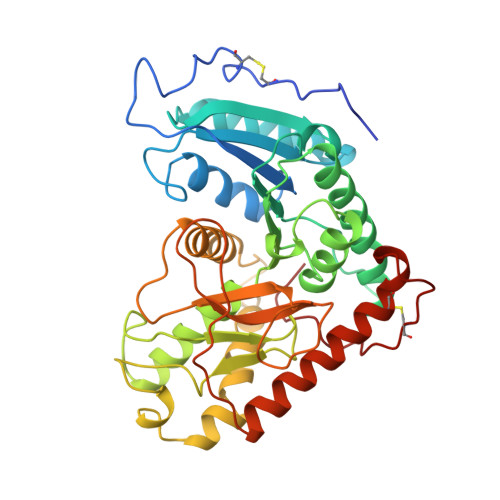
NADP-dependent malate dehydrogenase (EC 1.1.1.82) is a light-activated chloroplast enzyme that functions in the C4 pathway of photosynthesis. The light regulation is believed to be mediated in vivo by thioredoxin-catalyzed reduction and re-oxidation of cystine residues. The rates of reversible activation and inactivation of the enzyme are strongly influenced by the coenzyme substrates that seem to ultimately determine the steady-state extent of activation in vivo. The X-ray structure of the inactive, oxidized enzyme was determined at 2.8 A resolution. The core structure is homologous to AND-dependent malate dehydrogenases. Two surface-exposed and thioredoxin-accessible disulfide bonds are present, one in the N-terminal extension and the other in the C-terminal extension. The C-terminal peptide of the inactive, oxidized enzyme is constrained by its disulfide bond to fold into the active site over NADP+, hydrogen bonding to the catalytic His225 as well as obstructing access of the C4 acid substrate. Two loops flanking the active site, termed the Arg2 and Trp loops, that contain the C4 acid substrate binding residues are prevented from closing by the C-terminal extension. The structure explains the role of the C-terminal extension in inhibiting activity. The negative C terminus will interact more strongly with the positively charged nicotinamide of NADP+ than NADPH, explaining why the coenzyme-binding affinities of the enzyme differ so markedly from those of all other homologous alpha-hydroxy acid dehydrogenases. NADP+ may also slow dissociation of the C terminus upon reduction, providing a mechanism for the inhibition of activation by NADP+ but not NADPH.
Organizational Affiliation:
Research School of Chemistry, Australian National University, PO Box 414, Canberra ACT 2601, Australia. pdc@rsc.anu.edu.au