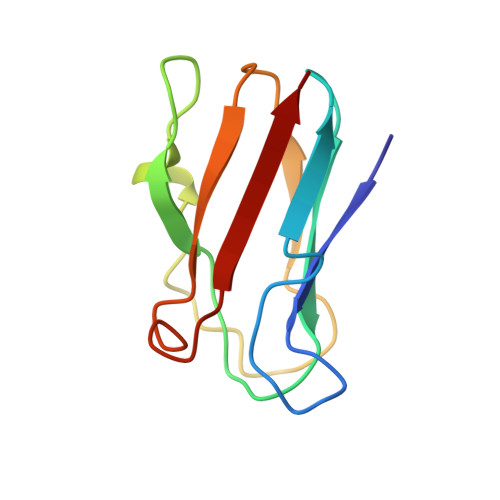
NMR solution structure of plastocyanin from the photosynthetic prokaryote, Prochlorothrix hollandica.
Babu, C.R., Volkman, B.F., Bullerjahn, G.S.(1999) Biochemistry 38: 4988-4995
- PubMed: 10213601
- DOI: https://doi.org/10.1021/bi983024f
- Primary Citation of Related Structures:
1B3I, 2B3I - PubMed Abstract:
The solution structure of a divergent plastocyanin (PC) from the photosynthetic prokaryote Prochlorothrix hollandica was determined by homonuclear 1H NMR spectroscopy. Nineteen structures were calculated from 1222 distance restraints, yielding a family of structures having an average rmsd of 0.42 +/- 0.08 A for backbone atoms and 0.71 +/- 0.07 A for heavy atoms to the mean structure. No distance constraint was violated by more than 0.26 A in the structure family. Despite the low number of conserved residues shared with other PC homologues, the overall folding pattern of P. hollandica PC is similar to other PCs, in that the protein forms a two-sheet beta-barrel tertiary structure. The greatest variability among the backbone structures is seen in the loop region from residues 47-60. The differences seen in the P. hollandica PC homologue likely arise due to a small deletion of 2-4 residues compared to the PC consensus; this yields a less extended loop containing a short alpha-helix from residues Ala52-Leu55. Additionally, the protein has an altered hydrophobic patch thought to be important in binding reaction partners. Whereas the backbone structure is very similar within the loops of the hydrophobic region, the presence of two unique residues (Tyr12 and Pro14) yields a structurally different hydrophobic surface likely important in binding P. hollandica Photosystem I.
Organizational Affiliation:
Center for Photochemical Sciences, Department of Biological Sciences, Bowling Green State University, OH 43403, USA.