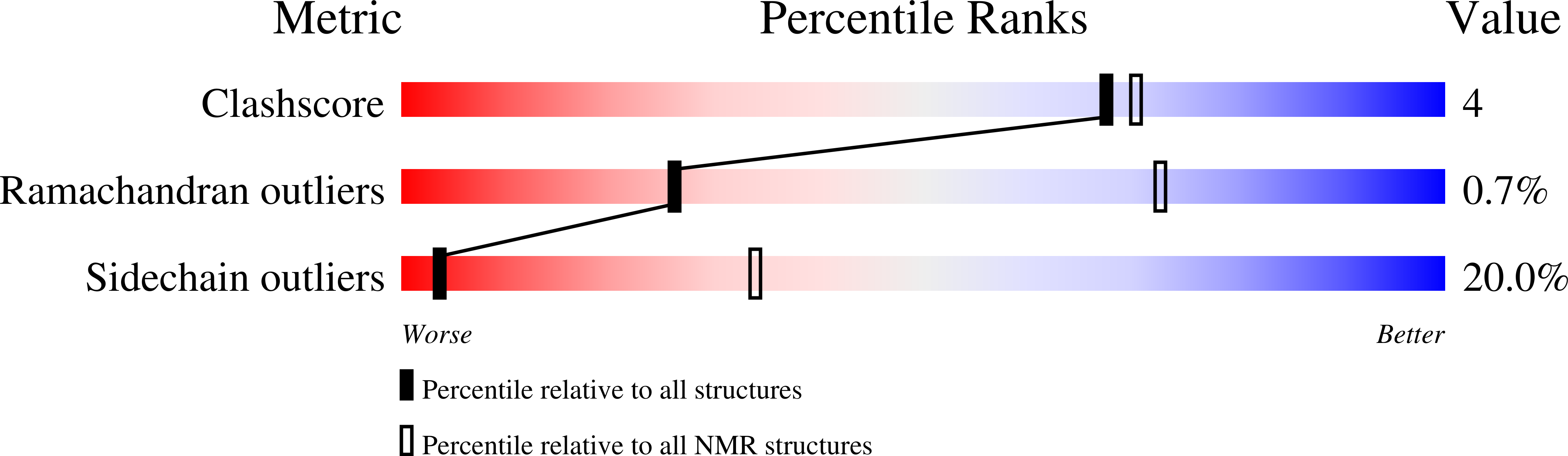Peptides derived from hookworm anti-inflammatory proteins suppress inducible colitis in mice and inflammatory cytokine production by human cells.
Cobos, C., Bansal, P.S., Wilson, D.T., Jones, L., Zhao, G., Field, M.A., Eichenberger, R.M., Pickering, D.A., Ryan, R.Y.M., Ratnatunga, C.N., Miles, J.J., Ruscher, R., Giacomin, P.R., Navarro, S., Loukas, A., Daly, N.L.(2022) Front Med (Lausanne) 9: 934852-934852
- PubMed: 36186812
- DOI: https://doi.org/10.3389/fmed.2022.934852
- Primary Citation of Related Structures:
7T6G - PubMed Abstract:
A decline in the prevalence of parasites such as hookworms appears to be correlated with the rise in non-communicable inflammatory conditions in people from high- and middle-income countries. This correlation has led to studies that have identified proteins produced by hookworms that can suppress inflammatory bowel disease (IBD) and asthma in animal models. Hookworms secrete a family of abundant netrin-domain containing proteins referred to as AIPs (Anti-Inflammatory Proteins), but there is no information on the structure-function relationships. Here we have applied a downsizing approach to the hookworm AIPs to derive peptides of 20 residues or less, some of which display anti-inflammatory effects when co-cultured with human peripheral blood mononuclear cells and oral therapeutic activity in a chemically induced mouse model of acute colitis. Our results indicate that a conserved helical region is responsible, at least in part, for the anti-inflammatory effects. This helical region has potential in the design of improved leads for treating IBD and possibly other inflammatory conditions.
Organizational Affiliation:
Centre for Molecular Therapeutics, Australian Institute of Tropical Health and Medicine, James Cook University, Cairns, QLD, Australia.