Negative charge of the AC-to-Hly linking segment modulates calcium-dependent membrane activities of Bordetella adenylate cyclase toxin.
Sukova, A., Bumba, L., Srb, P., Veverka, V., Stanek, O., Holubova, J., Chmelik, J., Fiser, R., Sebo, P., Masin, J.(2020) Biochim Biophys Acta Biomembr 1862: 183310-183310
- PubMed: 32333856
- DOI: https://doi.org/10.1016/j.bbamem.2020.183310
- Primary Citation of Related Structures:
6RFM - PubMed Abstract:
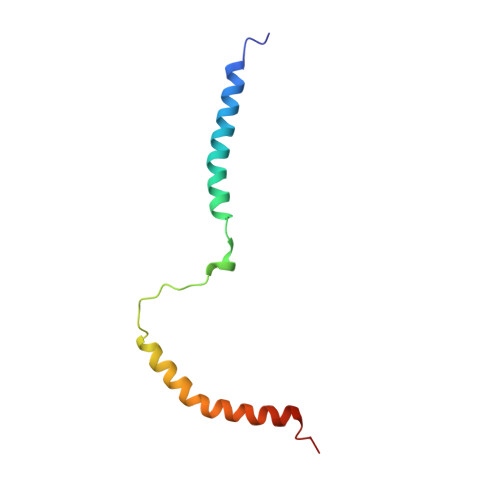
Two distinct conformers of the adenylate cyclase toxin (CyaA) appear to accomplish its two parallel activities within target cell membrane. The translocating conformer would deliver the N-terminal adenylyl cyclase (AC) enzyme domain across plasma membrane into cytosol of cells, while the pore precursor conformer would assemble into oligomeric cation-selective pores and permeabilize cellular membrane. Both toxin activities then involve a membrane-interacting 'AC-to-Hly-linking segment' (residues 400 to 500). Here, we report the NMR structure of the corresponding CyaA 411 - 490 polypeptide in dodecylphosphocholine micelles and show that it consists of two α-helices linked by an unrestrained loop. The N-terminal α-helix (Gly418 to His439) remained solvent accessible, while the C-terminal α-helix (His457 to Phe485) was fully enclosed within detergent micelles. CyaA 411 - 490 weakly bound Ca 2+ ions (apparent K D 2.6 mM) and permeabilized negatively charged lipid vesicles. At high concentrations (10 μM) the CyaA 411 - 490 polypeptide formed stable conductance units in artificial lipid bilayers with applied voltage, suggesting its possible transmembrane orientation in the membrane-inserted toxin. Mutagenesis revealed that two clusters of negatively charged residues within the 'AC-to-Hly-linking segment' (Glu419 to Glu432 and Asp445 to Glu448) regulate the balance between the AC domain translocating and pore-forming capacities of CyaA in function of calcium concentration.
Organizational Affiliation:
Institute of Microbiology of the Czech Academy of Sciences, Videnska 1083, 142 20 Prague, Czech Republic.