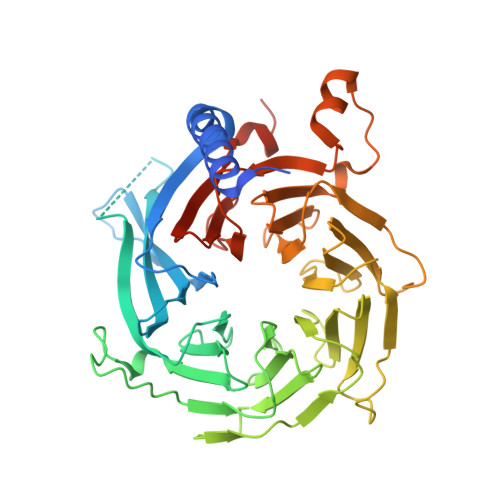
A Dimeric Structural Scaffold for PRC2-PCL Targeting to CpG Island Chromatin.
Chen, S., Jiao, L., Liu, X., Yang, X., Liu, X.(2020) Mol Cell 77: 1265
- PubMed: 31959557
- DOI: https://doi.org/10.1016/j.molcel.2019.12.019
- Primary Citation of Related Structures:
6NQ3 - PubMed Abstract:
Diverse accessory subunits are involved in the recruitment of polycomb repressive complex 2 (PRC2) to CpG island (CGI) chromatin. Here we report the crystal structure of a SUZ12-RBBP4 complex bound to fragments of the accessory subunits PHF19 and JARID2. Unexpectedly, this complex adopts a dimeric structural architecture, accounting for PRC2 self-association that has long been implicated. The intrinsic PRC2 dimer is formed via domain swapping involving RBBP4 and the unique C2 domain of SUZ12. MTF2 and PHF19 associate with PRC2 at around the dimer interface and stabilize the dimer. Conversely, AEBP2 binding results in a drastic movement of the C2 domain, disrupting the intrinsic PRC2 dimer. PRC2 dimerization enhances CGI DNA binding by PCLs in pairs in vitro, reminiscent of the widespread phenomenon of transcription factor dimerization in active transcription. Loss of PRC2 dimerization impairs histone H3K27 trimethylation (H3K27me3) on chromatin at developmental gene loci in mouse embryonic stem cells.
Organizational Affiliation:
Cecil H. and Ida Green Center for Reproductive Biology Sciences and Division of Basic Research, Department of Obstetrics and Gynecology, UT Southwestern Medical Center, Dallas, TX 75390, USA; Department of Biophysics, UT Southwestern Medical Center, Dallas, TX 75390, USA.