Structure and Conformational Dynamics of a COMPASS Histone H3K4 Methyltransferase Complex.
Qu, Q., Takahashi, Y.H., Yang, Y., Hu, H., Zhang, Y., Brunzelle, J.S., Couture, J.F., Shilatifard, A., Skiniotis, G.(2018) Cell 174: 1117-1126.e12
- PubMed: 30100186
- DOI: https://doi.org/10.1016/j.cell.2018.07.020
- Primary Citation of Related Structures:
6BX3, 6E29 - PubMed Abstract:
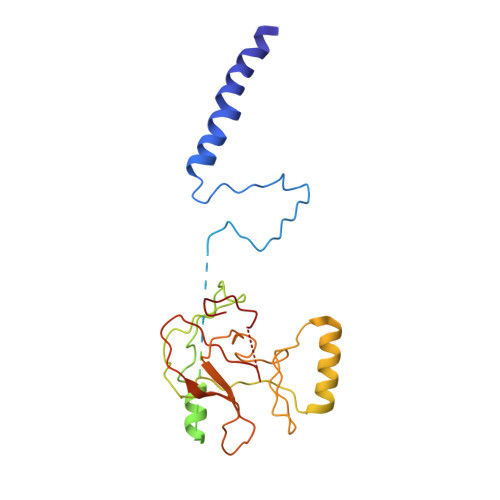
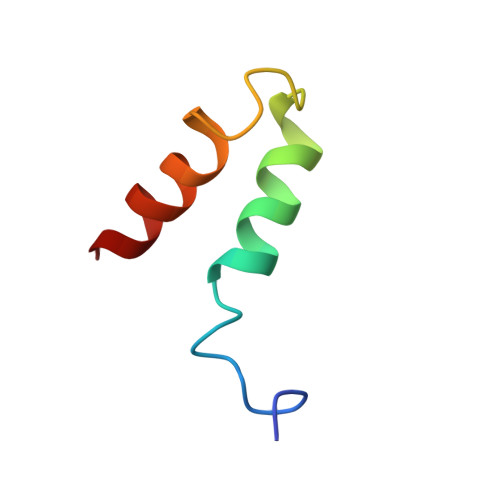
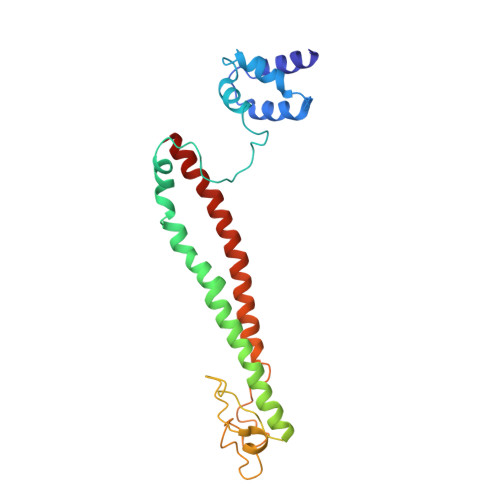
The methylation of histone 3 lysine 4 (H3K4) is carried out by an evolutionarily conserved family of methyltransferases referred to as complex of proteins associated with Set1 (COMPASS). The activity of the catalytic SET domain (su(var)3-9, enhancer-of-zeste, and trithorax) is endowed through forming a complex with a set of core proteins that are widely shared from yeast to humans. We obtained cryo-electron microscopy (cryo-EM) maps of the yeast Set1/COMPASS core complex at overall 4.0- to 4.4-Å resolution, providing insights into its structural organization and conformational dynamics. The Cps50 C-terminal tail weaves within the complex to provide a central scaffold for assembly. The SET domain, snugly positioned at the junction of the Y-shaped complex, is extensively contacted by Cps60 (Bre2), Cps50 (Swd1), and Cps30 (Swd3). The mobile SET-I motif of the SET domain is engaged by Cps30, explaining its key role in COMPASS catalytic activity toward higher H3K4 methylation states.
Organizational Affiliation:
Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA; Department of Structural Biology, Stanford University School of Medicine, Stanford, CA 94305, USA.