Msp1 Is a Membrane Protein Dislocase for Tail-Anchored Proteins.
Wohlever, M.L., Mateja, A., McGilvray, P.T., Day, K.J., Keenan, R.J.(2017) Mol Cell 67: 194-202.e6
- PubMed: 28712723
- DOI: https://doi.org/10.1016/j.molcel.2017.06.019
- Primary Citation of Related Structures:
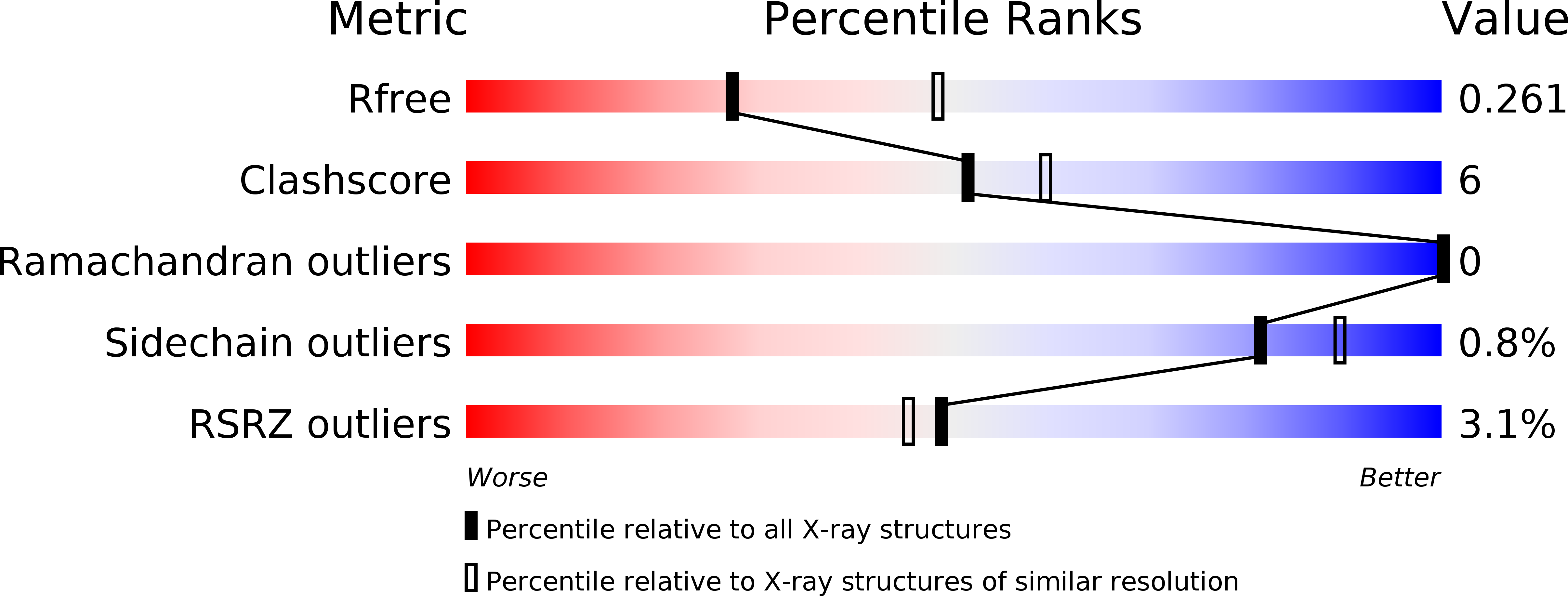
5W0T - PubMed Abstract:
Mislocalized tail-anchored (TA) proteins of the outer mitochondrial membrane are cleared by a newly identified quality control pathway involving the conserved eukaryotic protein Msp1 (ATAD1 in humans). Msp1 is a transmembrane AAA-ATPase, but its role in TA protein clearance is not known. Here, using purified components reconstituted into proteoliposomes, we show that Msp1 is both necessary and sufficient to drive the ATP-dependent extraction of TA proteins from the membrane. A crystal structure of the Msp1 cytosolic region modeled into a ring hexamer suggests that active Msp1 contains a conserved membrane-facing surface adjacent to a central pore. Structure-guided mutagenesis of the pore residues shows that they are critical for TA protein extraction in vitro and for functional complementation of an msp1 deletion in yeast. Together, these data provide a molecular framework for Msp1-dependent extraction of mislocalized TA proteins from the outer mitochondrial membrane.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, The University of Chicago, 929 East 57th Street, Chicago, IL 60637, USA.