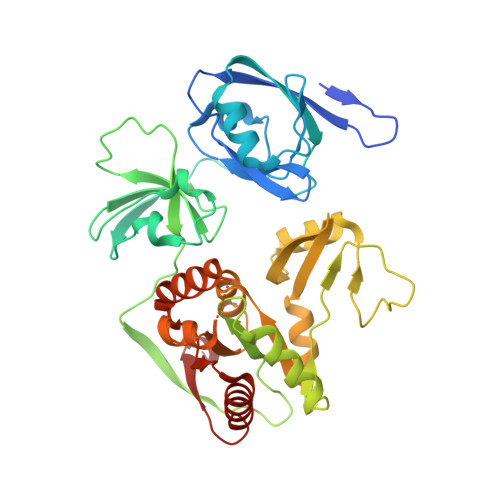
Structure of Crumbs tail in complex with the PALS1 PDZ-SH3-GK tandem reveals a highly specific assembly mechanism for the apical Crumbs complex.
Li, Y., Wei, Z., Yan, Y., Wan, Q., Du, Q., Zhang, M.(2014) Proc Natl Acad Sci U S A 111: 17444-17449
- PubMed: 25385611
- DOI: https://doi.org/10.1073/pnas.1416515111
- Primary Citation of Related Structures:
4WSI - PubMed Abstract:
The Crumbs (Crb) complex, formed by Crb, PALS1, and PATJ, is evolutionarily conserved in metazoans and acts as a master cell-growth and -polarity regulator at the apical membranes in polarized epithelia. Crb intracellular functions, including its direct binding to PALS1, are mediated by Crb's highly conserved 37-residue cytoplasmic tail. However, the mechanistic basis governing the highly specific Crb-PALS1 complex formation is unclear, as reported interaction between the Crb tail (Crb-CT) and PALS1 PSD-95/DLG/ZO-1 (PDZ) domain is weak and promiscuous. Here we have discovered that the PDZ-Src homolgy 3 (SH3)-Guanylate kinase (GK) tandem of PALS1 binds to Crb-CT with a dissociation constant of 70 nM, which is ∼ 100-fold stronger than the PALS1 PDZ-Crb-CT interaction. The crystal structure of the PALS1 PDZ-SH3-GK-Crb-CT complex reveals that PDZ-SH3-GK forms a structural supramodule with all three domains contributing to the tight binding to Crb. Mutations disrupting the tertiary interactions of the PDZ-SH3-GK supramodule weaken the PALS1-Crb interaction and compromise PALS1-mediated polarity establishment in Madin-Darby canine kidney (MDCK) cysts. We further show that specific target binding of other members of membrane-associated guanylate kinases (MAGUKs) (e.g., CASK binding to neurexin) also requires the presence of their PDZ-SH3-GK tandems.
Organizational Affiliation:
Division of Life Science, State Key Laboratory of Molecular Neuroscience and.