Bilateral Inhibition of Hausp Deubiquitinase by a Viral Interferon Regulatory Factor Protein
Lee, H.R., Choi, W.C., Lee, S., Hwang, J., Hwang, E., Guchhait, K., Haas, J., Toth, Z., Jeon, Y.H., Oh, T.K., Kim, M.H., Jung, J.U.(2011) Nat Struct Mol Biol 18: 1336
- PubMed: 22056774
- DOI: https://doi.org/10.1038/nsmb.2142
- Primary Citation of Related Structures:
2XXN - PubMed Abstract:
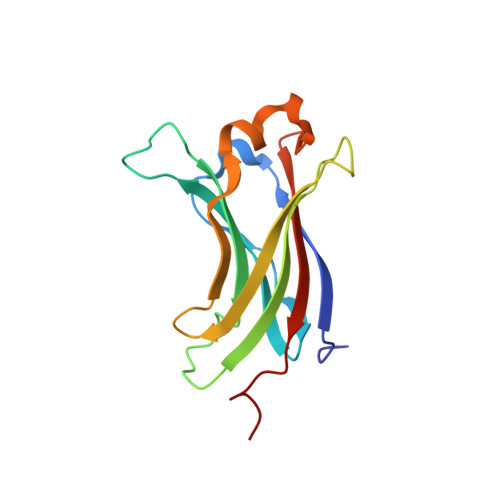
Herpesvirus-associated ubiquitin-specific protease (HAUSP) regulates the stability of p53 and the p53-binding protein MDM2, implicating HAUSP as a therapeutic target for tuning p53-mediated antitumor activity. Here we report the structural analysis of HAUSP with Kaposi's sarcoma-associated herpesvirus viral interferon (IFN) regulatory factor 4 (vIRF4) and the discovery of two vIRF4-derived peptides, vif1 and vif2, as potent and selective HAUSP antagonists. This analysis reveals a bilateral belt-type interaction that results in inhibition of HAUSP. The vif1 peptide binds the HAUSP TRAF domain, competitively blocking substrate binding, whereas the vif2 peptide binds both the HAUSP TRAF and catalytic domains, robustly suppressing its deubiquitination activity. Peptide treatments comprehensively blocked HAUSP, leading to p53-dependent cell-cycle arrest and apoptosis in culture and to tumor regression in xenograft mouse model. Thus, the virus has developed a unique strategy to target the HAUSP-MDM2-p53 pathway, and these virus-derived short peptides represent biologically active HAUSP antagonists.
Organizational Affiliation:
Department of Molecular Microbiology and Immunology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.