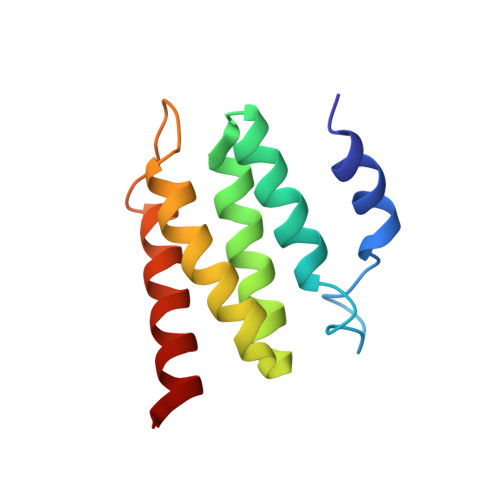
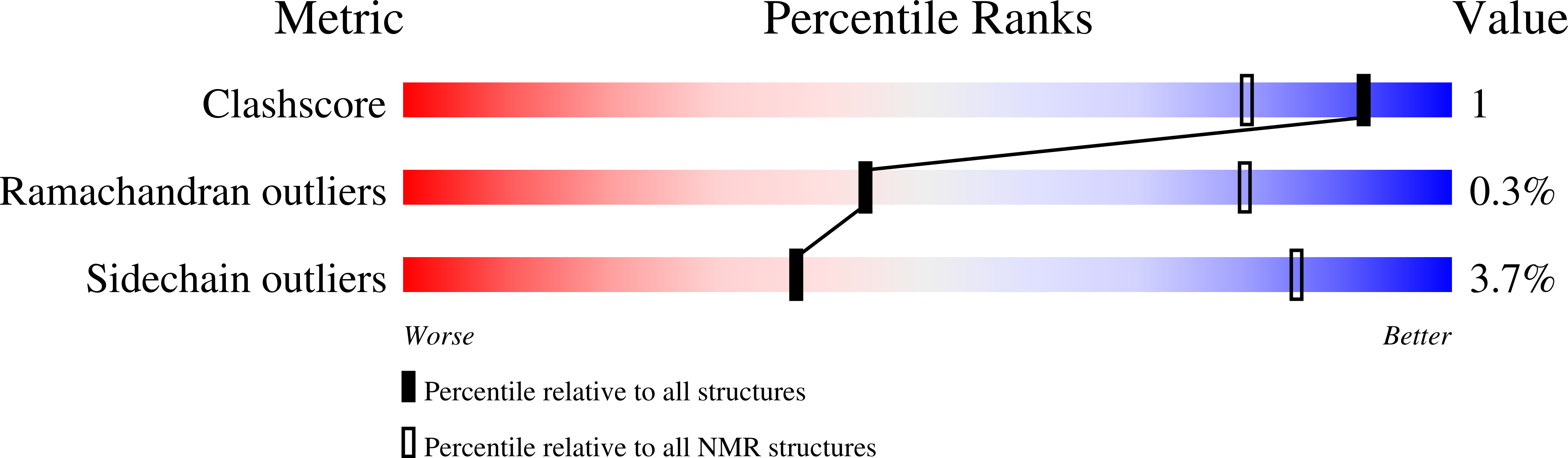Design of structurally distinct proteins using strategies inspired by evolution.
Jacobs, T.M., Williams, B., Williams, T., Xu, X., Eletsky, A., Federizon, J.F., Szyperski, T., Kuhlman, B.(2016) Science 352: 687-690
- PubMed: 27151863
- DOI: https://doi.org/10.1126/science.aad8036
- Primary Citation of Related Structures:
2N8I, 2N8W, 5E6G - PubMed Abstract:
Natural recombination combines pieces of preexisting proteins to create new tertiary structures and functions. We describe a computational protocol, called SEWING, which is inspired by this process and builds new proteins from connected or disconnected pieces of existing structures. Helical proteins designed with SEWING contain structural features absent from other de novo designed proteins and, in some cases, remain folded at more than 100°C. High-resolution structures of the designed proteins CA01 and DA05R1 were solved by x-ray crystallography (2.2 angstrom resolution) and nuclear magnetic resonance, respectively, and there was excellent agreement with the design models. This method provides a new strategy to rapidly create large numbers of diverse and designable protein scaffolds.
Organizational Affiliation:
Program in Bioinformatics and Computational Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.