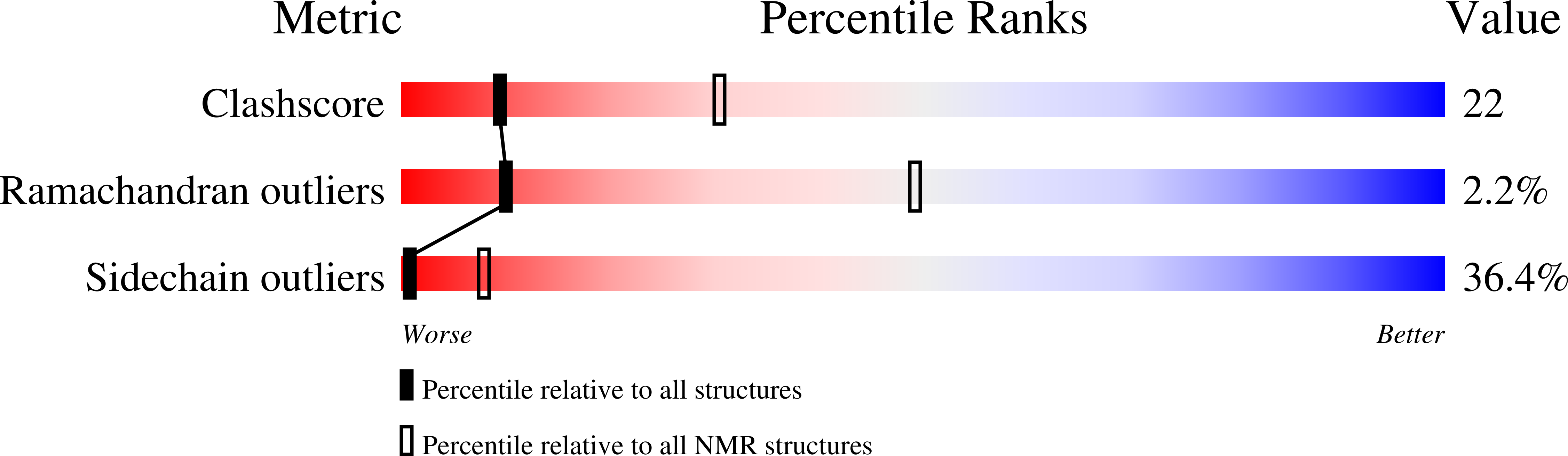Photochemical tyrosine oxidation in the structurally well-defined alpha 3Y protein: proton-coupled electron transfer and a long-lived tyrosine radical.
Glover, S.D., Jorge, C., Liang, L., Valentine, K.G., Hammarstrom, L., Tommos, C.(2014) J Am Chem Soc 136: 14039-14051
- PubMed: 25121576
- DOI: https://doi.org/10.1021/ja503348d
- Primary Citation of Related Structures:
2MI7 - PubMed Abstract:
Tyrosine oxidation-reduction involves proton-coupled electron transfer (PCET) and a reactive radical state. These properties are effectively controlled in enzymes that use tyrosine as a high-potential, one-electron redox cofactor. The α3Y model protein contains Y32, which can be reversibly oxidized and reduced in voltammetry measurements. Structural and kinetic properties of α3Y are presented. A solution NMR structural analysis reveals that Y32 is the most deeply buried residue in α3Y. Time-resolved spectroscopy using a soluble flash-quench generated [Ru(2,2'-bipyridine)3](3+) oxidant provides high-quality Y32-O• absorption spectra. The rate constant of Y32 oxidation (kPCET) is pH dependent: 1.4 × 10(4) M(-1) s(-1) (pH 5.5), 1.8 × 10(5) M(-1) s(-1) (pH 8.5), 5.4 × 10(3) M(-1) s(-1) (pD 5.5), and 4.0 × 10(4) M(-1) s(-1) (pD 8.5). k(H)/k(D) of Y32 oxidation is 2.5 ± 0.5 and 4.5 ± 0.9 at pH(D) 5.5 and 8.5, respectively. These pH and isotope characteristics suggest a concerted or stepwise, proton-first Y32 oxidation mechanism. The photochemical yield of Y32-O• is 28-58% versus the concentration of [Ru(2,2'-bipyridine)3](3+). Y32-O• decays slowly, t1/2 in the range of 2-10 s, at both pH 5.5 and 8.5, via radical-radical dimerization as shown by second-order kinetics and fluorescence data. The high stability of Y32-O• is discussed relative to the structural properties of the Y32 site. Finally, the static α3Y NMR structure cannot explain (i) how the phenolic proton released upon oxidation is removed or (ii) how two Y32-O• come together to form dityrosine. These observations suggest that the dynamic properties of the protein ensemble may play an essential role in controlling the PCET and radical decay characteristics of α3Y.
Organizational Affiliation:
Department of Chemistry, Ångström Laboratory, Uppsala University , Box 523, SE75120 Uppsala, Sweden.