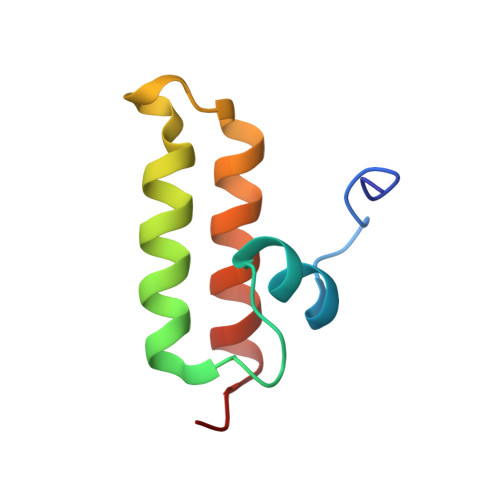
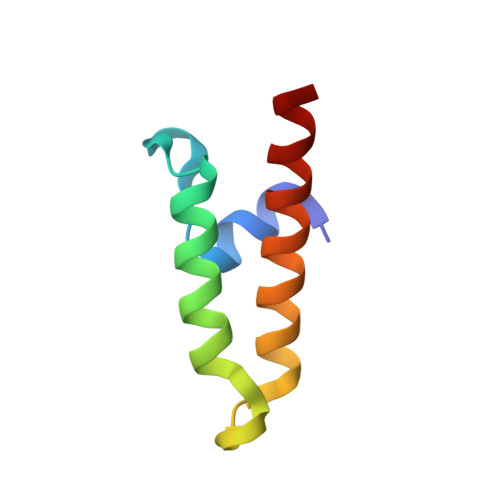
Structure and function of Tim14 and Tim16, the J and J-like components of the mitochondrial protein import motor.
Mokranjac, D., Bourenkov, G., Hell, K., Neupert, W., Groll, M.(2006) EMBO J 25: 4675-4685
- PubMed: 16977310
- DOI: https://doi.org/10.1038/sj.emboj.7601334
- Primary Citation of Related Structures:
2GUZ - PubMed Abstract:
The import motor of the mitochondrial translocase of the inner membrane (TIM23) mediates the ATP-dependent translocation of preproteins into the mitochondrial matrix by cycles of binding to and release from mtHsp70. An essential step of this process is the stimulation of the ATPase activity of mtHsp70 performed by the J cochaperone Tim14. Tim14 forms a complex with the J-like protein Tim16. The crystal structure of this complex shows that the conserved domains of the two proteins have virtually identical folds but completely different surfaces enabling them to perform different functions. The Tim14-Tim16 dimer reveals a previously undescribed arrangement of J and J-like domains. Mutations that destroy the complex between Tim14 and Tim16 are lethal demonstrating that complex formation is an essential requirement for the viability of cells. We further demonstrate tight regulation of the cochaperone activity of Tim14 by Tim16. The first crystal structure of a J domain in complex with a regulatory protein provides new insights into the function of the mitochondrial TIM23 translocase and the Hsp70 chaperone system in general.
Organizational Affiliation:
Institute for Physiological Chemistry, Ludwig-Maximilians University, Munich, Germany.