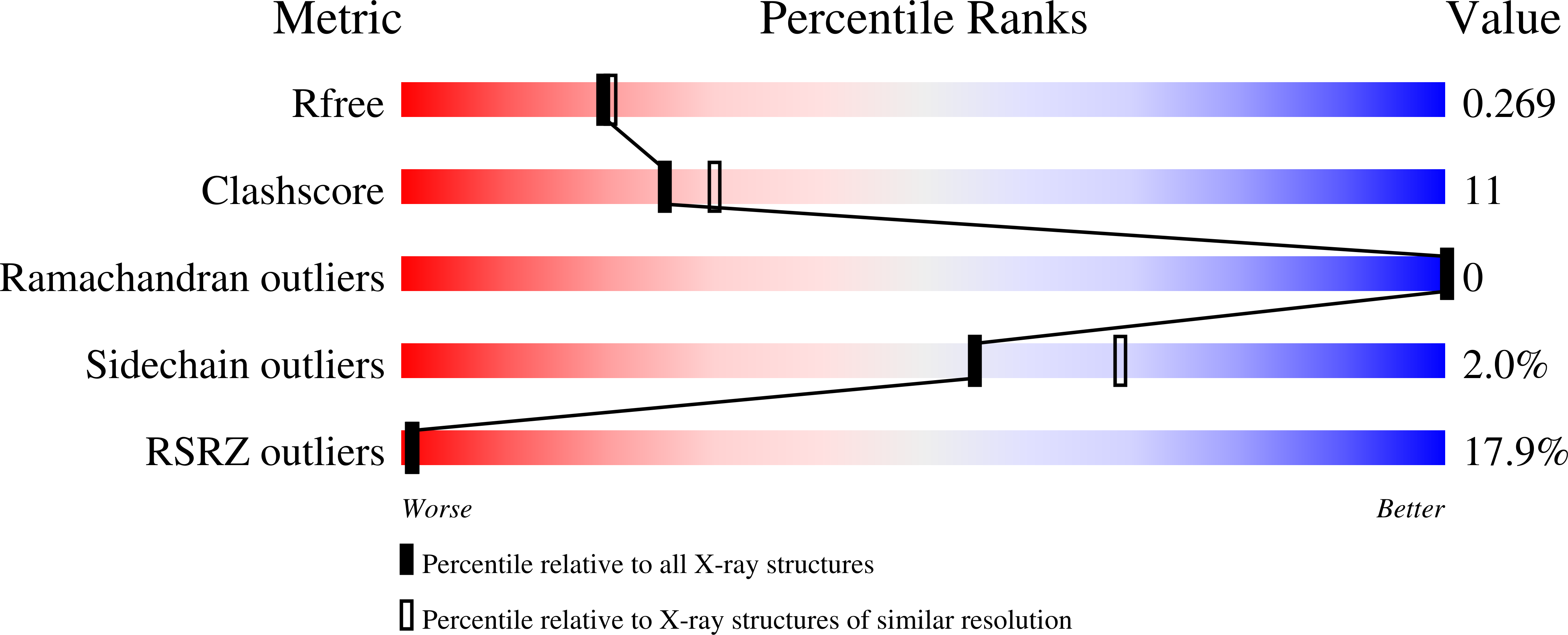
The Structure of Bovine If(1), the Regulatory Subunit of Mitochondrial F-ATPase.
Cabezon, E., Runswick, M.J., Leslie, A.G.W., Walker, J.E.(2001) EMBO J 20: 6990
- PubMed: 11742976
- DOI: https://doi.org/10.1093/emboj/20.24.6990
- Primary Citation of Related Structures:
1GMJ - PubMed Abstract:
In mitochondria, the hydrolytic activity of ATP synthase is regulated by an inhibitor protein, IF(1). Its binding to ATP synthase depends on pH, and below neutrality, IF(1) is dimeric and forms a stable complex with the enzyme. At higher pH values, IF(1) forms tetramers and is inactive. In the 2.2 A structure of the bovine IF(1) described here, the four monomers in the asymmetric unit are arranged as a dimer of dimers. Monomers form dimers via an antiparallel alpha-helical coiled coil in the C-terminal region. Dimers are associated into oligomers and form long fibres in the crystal lattice, via coiled-coil interactions in the N-terminal and inhibitory regions (residues 14-47). Therefore, tetramer formation masks the inhibitory region, preventing IF(1) binding to ATP synthase.
Organizational Affiliation:
The Medical Research Council Dunn Human Nutrition Unit, Hills Road, Cambridge CB2 2XY, UK.