Divergence of PUF protein specificity through variations in an RNA-binding pocket
Qiu, C., Kershner, A., Wang, Y., Holley, C.H., Wilinski, D., Keles, S., Kimble, J., Wickens, M., Hall, T.M.T.(2012) J Biol Chem
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2012) J Biol Chem
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fem-3 mRNA-binding factor 2 | 413 | Caenorhabditis elegans | Mutation(s): 0 Gene Names: fbf-2, F21H12.5 | 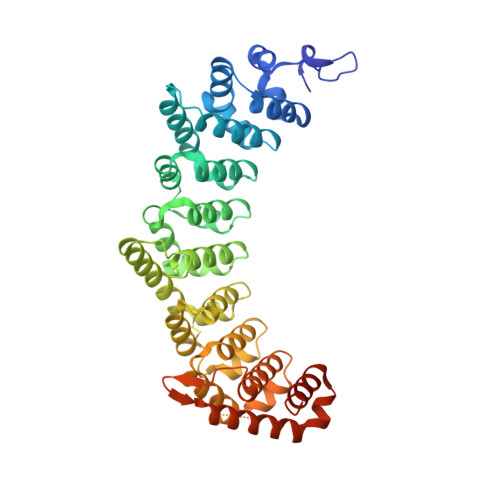 | |
UniProt | |||||
Find proteins for Q09312 (Caenorhabditis elegans) Explore Q09312 Go to UniProtKB: Q09312 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q09312 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| RNA (5'-R(*UP*AP*CP*UP*GP*UP*GP*CP*CP*AP*UP*AP*C)-3') | 13 | Caenorhabditis elegans | 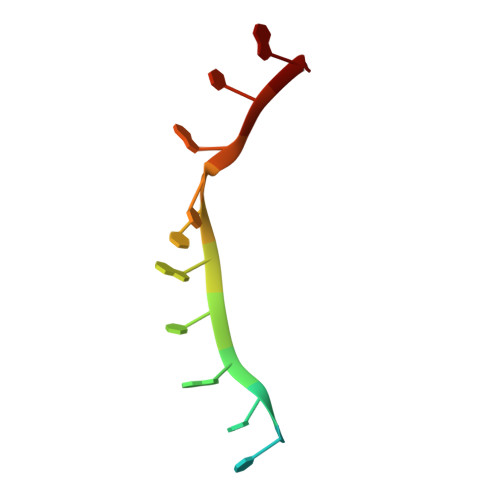 | ||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 99.797 | α = 90 |
| b = 99.797 | β = 90 |
| c = 105.598 | γ = 120 |
| Software Name | Purpose |
|---|---|
| HKL-2000 | data collection |
| PHASER | phasing |
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |