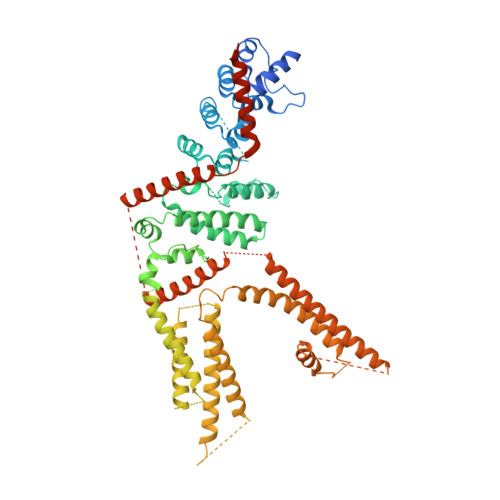
Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating.
Sierra-Valdez, F., Azumaya, C.M., Romero, L.O., Nakagawa, T., Cordero-Morales, J.F.(2018) J Biol Chem 293: 16102-16114
- PubMed: 30139744
- DOI: https://doi.org/10.1074/jbc.RA118.005066
- Primary Citation of Related Structures:
6D7L, 6DJR, 6DJS - PubMed Abstract:
The transient receptor potential ion channels support Ca 2+ permeation in many organs, including the heart, brain, and kidney. Genetic mutations in transient receptor potential cation channel subfamily C member 3 (TRPC3) are associated with neurodegenerative diseases, memory loss, and hypertension. To better understand the conformational changes that regulate TRPC3 function, we solved the cryo-EM structures for the full-length human TRPC3 and its cytoplasmic domain (CPD) in the apo state at 5.8- and 4.0-Å resolution, respectively. These structures revealed that the TRPC3 transmembrane domain resembles those of other TRP channels and that the CPD is a stable module involved in channel assembly and gating. We observed the presence of a C-terminal domain swap at the center of the CPD where horizontal helices (HHs) transition into a coiled-coil bundle. Comparison of TRPC3 structures revealed that the HHs can reside in two distinct positions. Electrophysiological analyses disclosed that shortening the length of the C-terminal loop connecting the HH with the TRP helices increases TRPC3 activity and that elongating the length of the loop has the opposite effect. Our findings indicate that the C-terminal loop affects channel gating by altering the allosteric coupling between the cytoplasmic and transmembrane domains. We propose that molecules that target the HH may represent a promising strategy for controlling TRPC3-associated neurological disorders and hypertension.
Organizational Affiliation:
From the Department of Physiology, University of Tennessee Health Science Center, Memphis, Tennessee 38163 and.