Enzymatic and Structural Characterization of the Major Endopeptidase in the Venus Flytrap Digestion Fluid.
Risor, M.W., Thomsen, L.R., Sanggaard, K.W., Nielsen, T.A., Thogersen, I.B., Lukassen, M.V., Rossen, L., Garcia-Ferrer, I., Guevara, T., Scavenius, C., Meinjohanns, E., Gomis-Ruth, F.X., Enghild, J.J.(2016) J Biol Chem 291: 2271
- PubMed: 26627834
- DOI: https://doi.org/10.1074/jbc.M115.672550
- Primary Citation of Related Structures:
5A24 - PubMed Abstract:
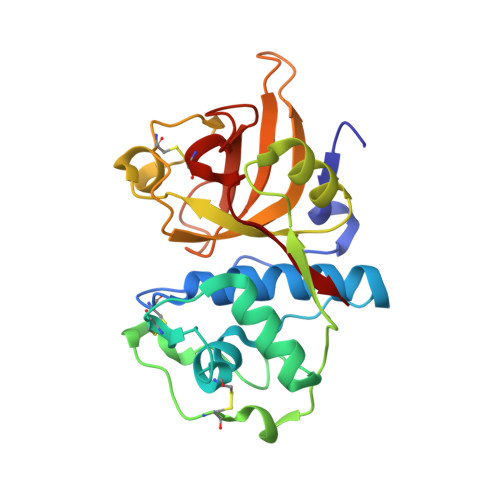
Carnivorous plants primarily use aspartic proteases during digestion of captured prey. In contrast, the major endopeptidases in the digestive fluid of the Venus flytrap (Dionaea muscipula) are cysteine proteases (dionain-1 to -4). Here, we present the crystal structure of mature dionain-1 in covalent complex with inhibitor E-64 at 1.5 Å resolution. The enzyme exhibits an overall protein fold reminiscent of other plant cysteine proteases. The inactive glycosylated pro-form undergoes autoprocessing and self-activation, optimally at the physiologically relevant pH value of 3.6, at which the protective effect of the pro-domain is lost. The mature enzyme was able to efficiently degrade a Drosophila fly protein extract at pH 4 showing high activity against the abundant Lys- and Arg-rich protein, myosin. The substrate specificity of dionain-1 was largely similar to that of papain with a preference for hydrophobic and aliphatic residues in subsite S2 and for positively charged residues in S1. A tentative structure of the pro-domain was obtained by homology modeling and suggested that a pro-peptide Lys residue intrudes into the S2 pocket, which is more spacious than in papain. This study provides the first analysis of a cysteine protease from the digestive fluid of a carnivorous plant and confirms the close relationship between carnivorous action and plant defense mechanisms.
Organizational Affiliation:
From the Department of Molecular Biology and Genetics, Aarhus University, DK-8000 Aarhus, Denmark, the Interdisciplinary Nanoscience Center (iNANO), DK-8000 Aarhus, Denmark.