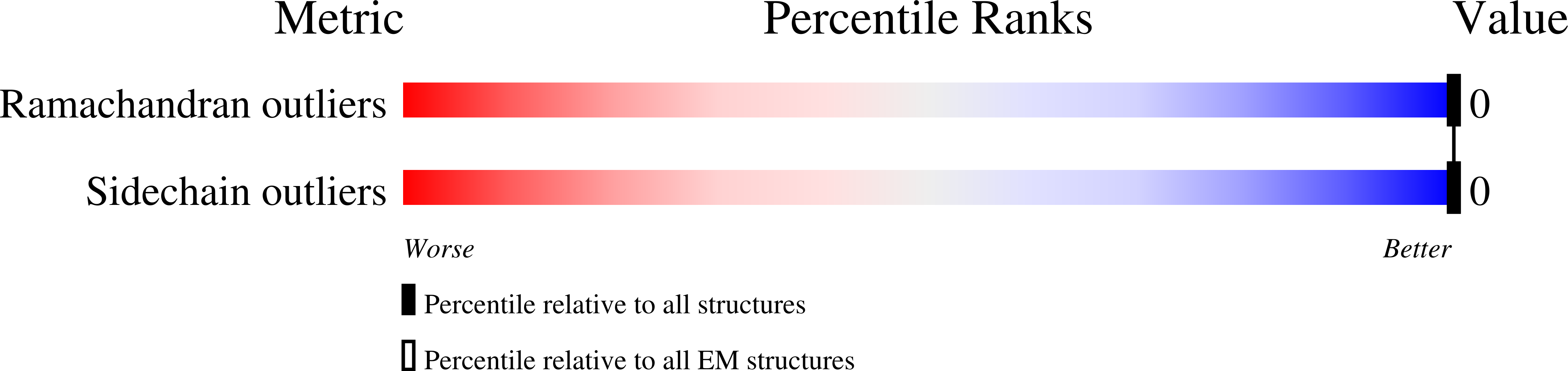Structural analysis of cross alpha-helical nanotubes provides insight into the designability of filamentous peptide nanomaterials.
Wang, F., Gnewou, O., Modlin, C., Beltran, L.C., Xu, C., Su, Z., Juneja, P., Grigoryan, G., Egelman, E.H., Conticello, V.P.(2021) Nat Commun 12: 407-407
- PubMed: 33462223
- DOI: https://doi.org/10.1038/s41467-020-20689-w
- Primary Citation of Related Structures:
6WKX, 6WKY, 6WL0, 6WL1, 6WL7, 6WL8, 6WL9 - PubMed Abstract:
The exquisite structure-function correlations observed in filamentous protein assemblies provide a paradigm for the design of synthetic peptide-based nanomaterials. However, the plasticity of quaternary structure in sequence-space and the lability of helical symmetry present significant challenges to the de novo design and structural analysis of such filaments. Here, we describe a rational approach to design self-assembling peptide nanotubes based on controlling lateral interactions between protofilaments having an unusual cross-α supramolecular architecture. Near-atomic resolution cryo-EM structural analysis of seven designed nanotubes provides insight into the designability of interfaces within these synthetic peptide assemblies and identifies a non-native structural interaction based on a pair of arginine residues. This arginine clasp motif can robustly mediate cohesive interactions between protofilaments within the cross-α nanotubes. The structure of the resultant assemblies can be controlled through the sequence and length of the peptide subunits, which generates synthetic peptide filaments of similar dimensions to flagella and pili.
Organizational Affiliation:
Department of Biochemistry and Molecular Genetics, University of Virginia, Charlottesville, VA, 22908, USA.