The structure-function relationship of oncogenic LMTK3.
Ditsiou, A., Cilibrasi, C., Simigdala, N., Papakyriakou, A., Milton-Harris, L., Vella, V., Nettleship, J.E., Lo, J.H., Soni, S., Smbatyan, G., Ntavelou, P., Gagliano, T., Iachini, M.C., Khurshid, S., Simon, T., Zhou, L., Hassell-Hart, S., Carter, P., Pearl, L.H., Owen, R.L., Owens, R.J., Roe, S.M., Chayen, N.E., Lenz, H.J., Spencer, J., Prodromou, C., Klinakis, A., Stebbing, J., Giamas, G.(2020) Sci Adv 6
- PubMed: 33188023
- DOI: https://doi.org/10.1126/sciadv.abc3099
- Primary Citation of Related Structures:
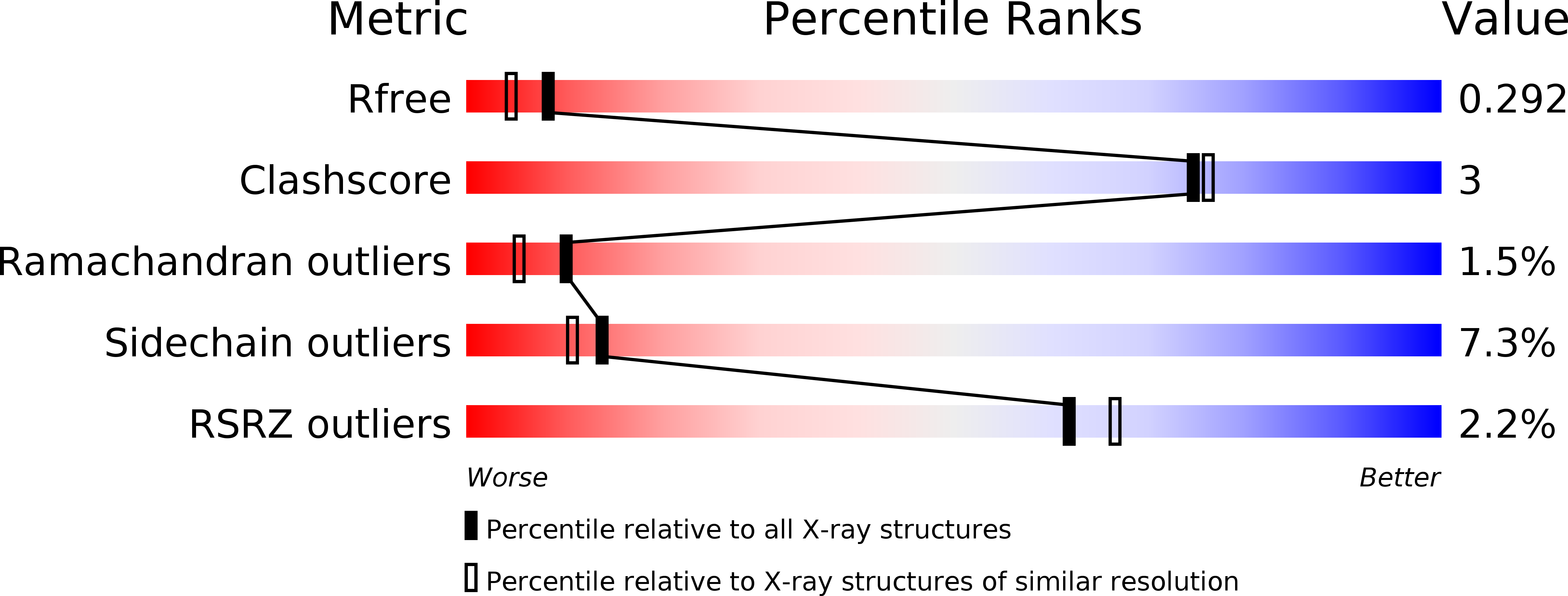
6SEQ - PubMed Abstract:
Elucidating signaling driven by lemur tyrosine kinase 3 (LMTK3) could help drug development. Here, we solve the crystal structure of LMTK3 kinase domain to 2.1Å resolution, determine its consensus motif and phosphoproteome, unveiling in vitro and in vivo LMTK3 substrates. Via high-throughput homogeneous time-resolved fluorescence screen coupled with biochemical, cellular, and biophysical assays, we identify a potent LMTK3 small-molecule inhibitor (C28). Functional and mechanistic studies reveal LMTK3 is a heat shock protein 90 (HSP90) client protein, requiring HSP90 for folding and stability, while C28 promotes proteasome-mediated degradation of LMTK3. Pharmacologic inhibition of LMTK3 decreases proliferation of cancer cell lines in the NCI-60 panel, with a concomitant increase in apoptosis in breast cancer cells, recapitulating effects of LMTK3 gene silencing. Furthermore, LMTK3 inhibition reduces growth of xenograft and transgenic breast cancer mouse models without displaying systemic toxicity at effective doses. Our data reinforce LMTK3 as a druggable target for cancer therapy.
Organizational Affiliation:
Department of Biochemistry and Biomedicine, School of Life Sciences, University of Sussex, Falmer, Brighton BN1 9QG, UK.