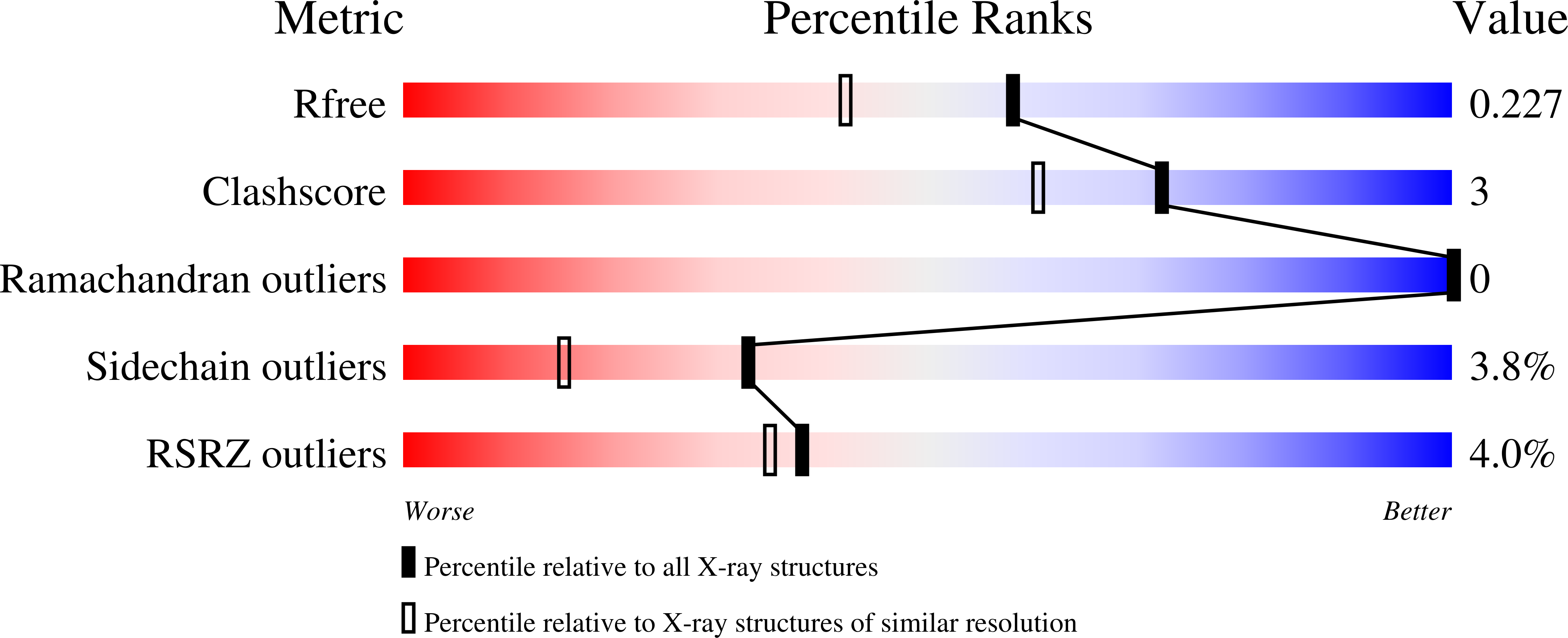
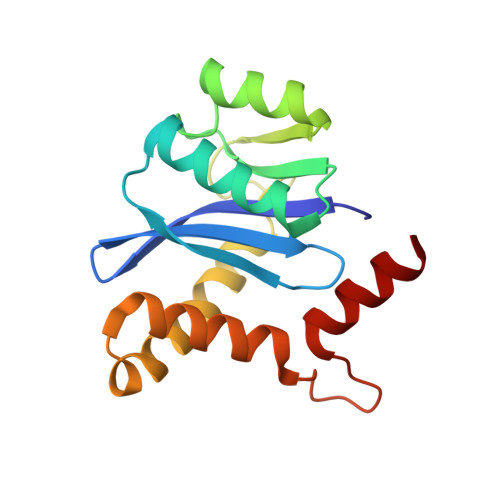
Identification of phe187 as a crucial dimerization determinant facilitates crystallization of a monomeric retroviral integrase core domain.
Galilee, M., Alian, A.(2014) Structure 22: 1512-1519
- PubMed: 25199694
- DOI: https://doi.org/10.1016/j.str.2014.08.001
- Primary Citation of Related Structures:
4MQ3, 4PA1 - PubMed Abstract:
Retroviral DNA integration into the host genome is mediated by nucleoprotein assemblies containing tetramers of viral integrase (IN). Whereas the fully active form of IN comprises a dimer of dimers, the molecular basis of IN multimerization has not been fully characterized. IN has consistently been crystallized in an analogous dimeric form in all crystallographic structures and experimental evidence as to the level of similarity between IN monomeric and dimeric conformations is missing because of the lack of IN monomeric structures. Here we identify Phe187 as a critical dimerization determinant of IN from feline immunodeficiency virus (FIV), a nonprimate lentivirus that causes AIDS in the natural host, and report, in addition to a canonical dimeric structure of the FIV IN core-domain, a monomeric structure revealing the preservation of the backbone structure between the two multimeric forms and suggest a role for Phe187 in "hinging" the flexible IN dimer.
Organizational Affiliation:
Faculty of Biology, Technion-Israel Institute of Technology, Haifa 320003, Israel.