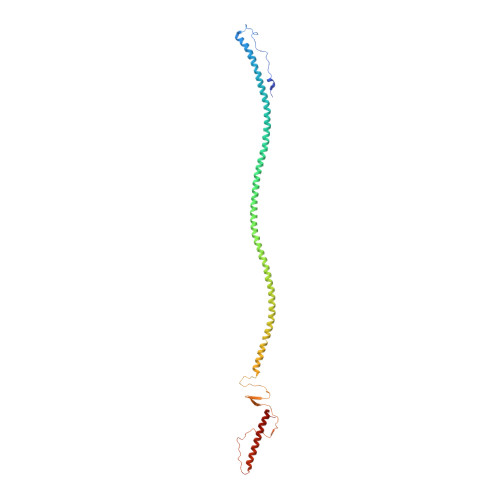
Exploring the atomic structure and conformational flexibility of a 320 angstrom long engineered viral fiber using X-ray crystallography.
Bhardwaj, A., Casjens, S.R., Cingolani, G.(2014) Acta Crystallogr D Biol Crystallogr 70: 342-353
- PubMed: 24531468
- DOI: https://doi.org/10.1107/S1399004713027685
- Primary Citation of Related Structures:
4LIN - PubMed Abstract:
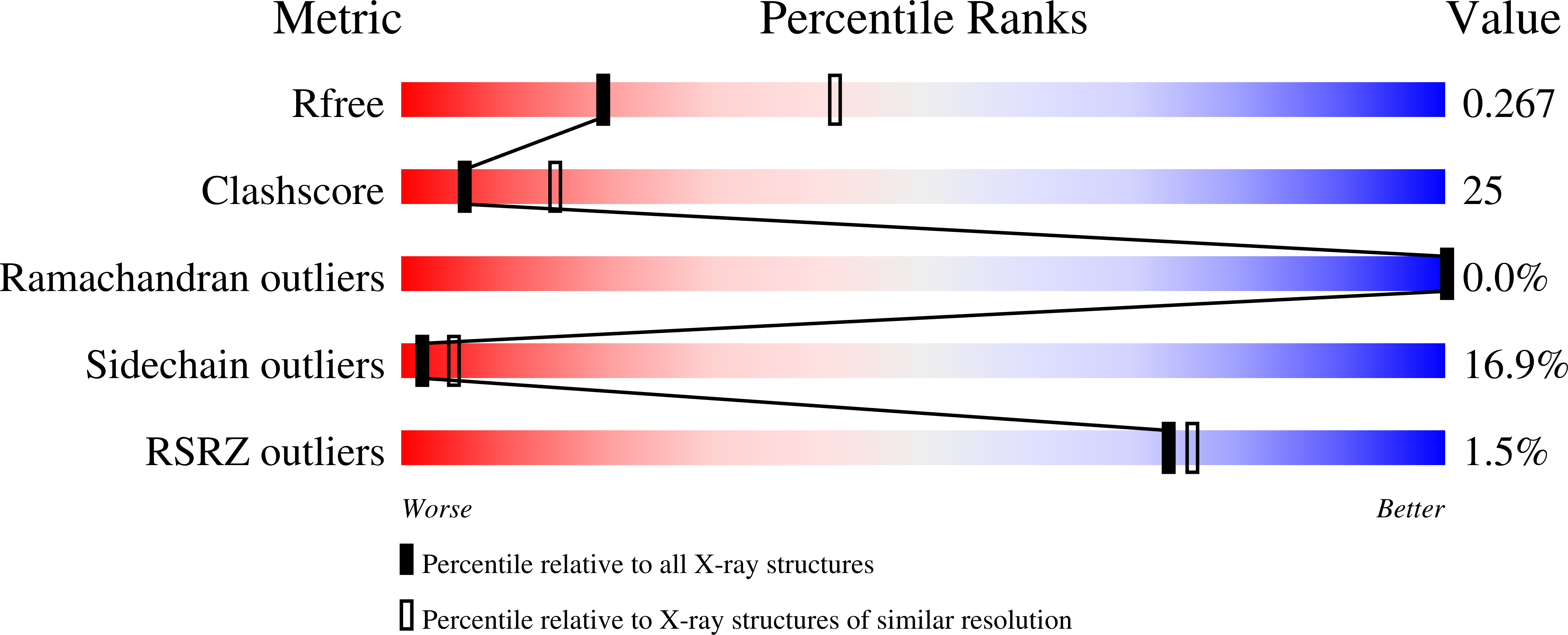
Protein fibers are widespread in nature, but only a limited number of high-resolution structures have been determined experimentally. Unlike globular proteins, fibers are usually recalcitrant to form three-dimensional crystals, preventing single-crystal X-ray diffraction analysis. In the absence of three-dimensional crystals, X-ray fiber diffraction is a powerful tool to determine the internal symmetry of a fiber, but it rarely yields atomic resolution structural information on complex protein fibers. An 85-residue-long minimal coiled-coil repeat unit (MiCRU) was previously identified in the trimeric helical core of tail needle gp26, a fibrous protein emanating from the tail apparatus of the bacteriophage P22 virion. Here, evidence is provided that an MiCRU can be inserted in frame inside the gp26 helical core to generate a rationally extended fiber (gp26-2M) which, like gp26, retains a trimeric quaternary structure in solution. The 2.7 Å resolution crystal structure of this engineered fiber, which measures ∼320 Å in length and is only 20-35 Å wide, was determined. This structure, the longest for a trimeric protein fiber to be determined to such a high resolution, reveals the architecture of 22 consecutive trimerization heptads and provides a framework to decipher the structural determinants for protein fiber assembly, stability and flexibility.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, Thomas Jefferson University, 233 South 10th Street, Philadelphia, PA 19107, USA.