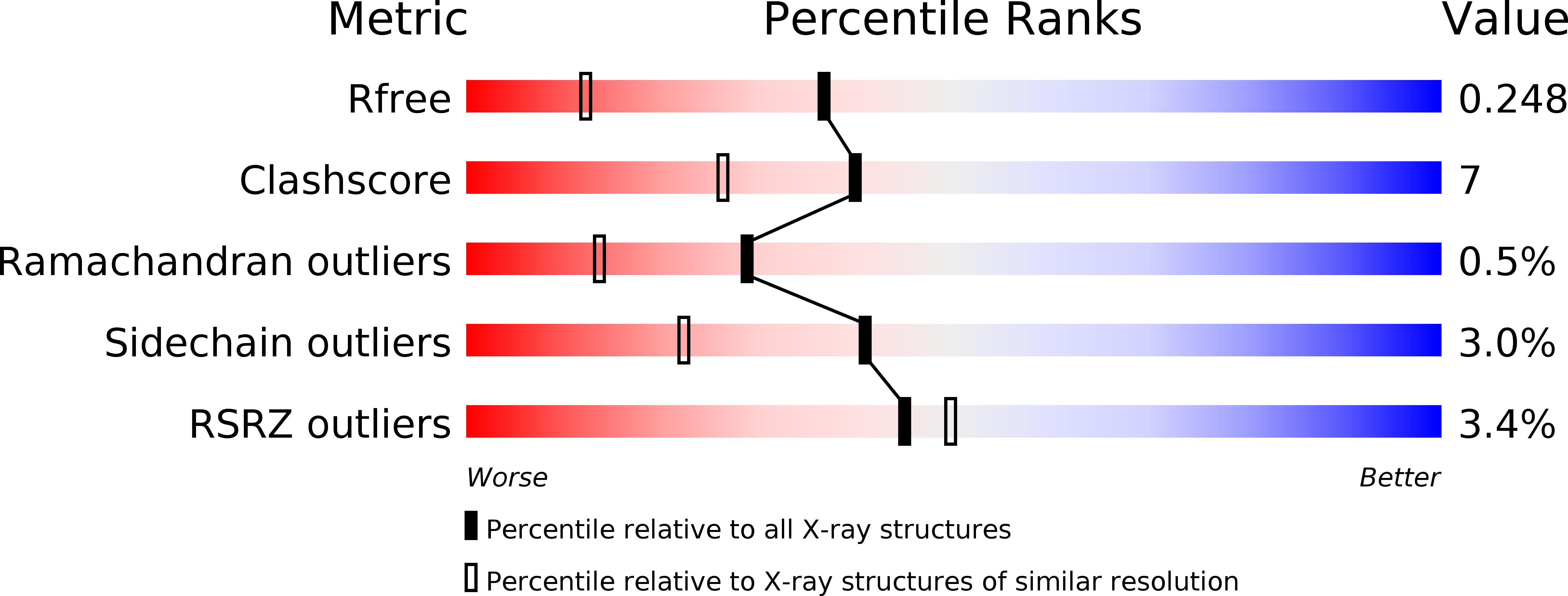
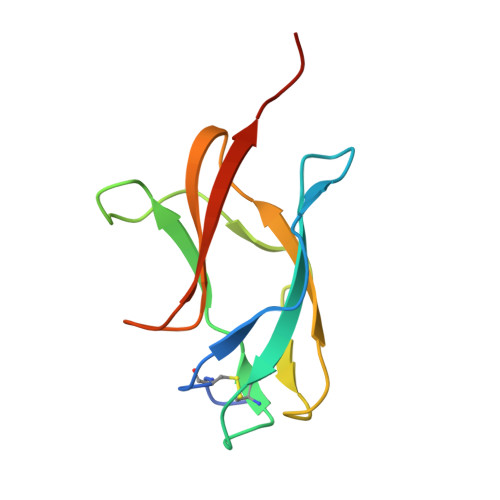
High resolution crystal structure of dengue-3 envelope protein domain III suggests possible molecular mechanisms for serospecific antibody recognition
Elahi, M., Islam, M.M., Noguchi, K., Yohda, M., Kuroda, Y.(2013) Proteins 81: 1090-1095
- PubMed: 23239402
- DOI: https://doi.org/10.1002/prot.24237
- Primary Citation of Related Structures:
3VTT - PubMed Abstract:
Dengue viruses are classified into four serotypes. Here, we report a 1.7 Å crystal structure of a recombinant dengue-3 envelope protein domain III (ED3), which contains most of the putative epitopes. Although the fold was well conserved, we found that a local backbone deformation in the first β-strand, which contains the putative epitope-1, occurred upon domain isolation. Furthermore, a comparison with dengue-2 ED3 indicated a large structural change by as much as 4.0 Å at Asp(662), located in epitope-2. These minute structural and surface properties changes observed in the high resolution ED3 structure represent potential determinants for serospecificity and epitope recognition by antibodies.
Organizational Affiliation:
Department of Biotechnology and Life Science, Graduate School of Engineering, Tokyo University of Agriculture and Technology, Koganei-shi, Tokyo, Japan.