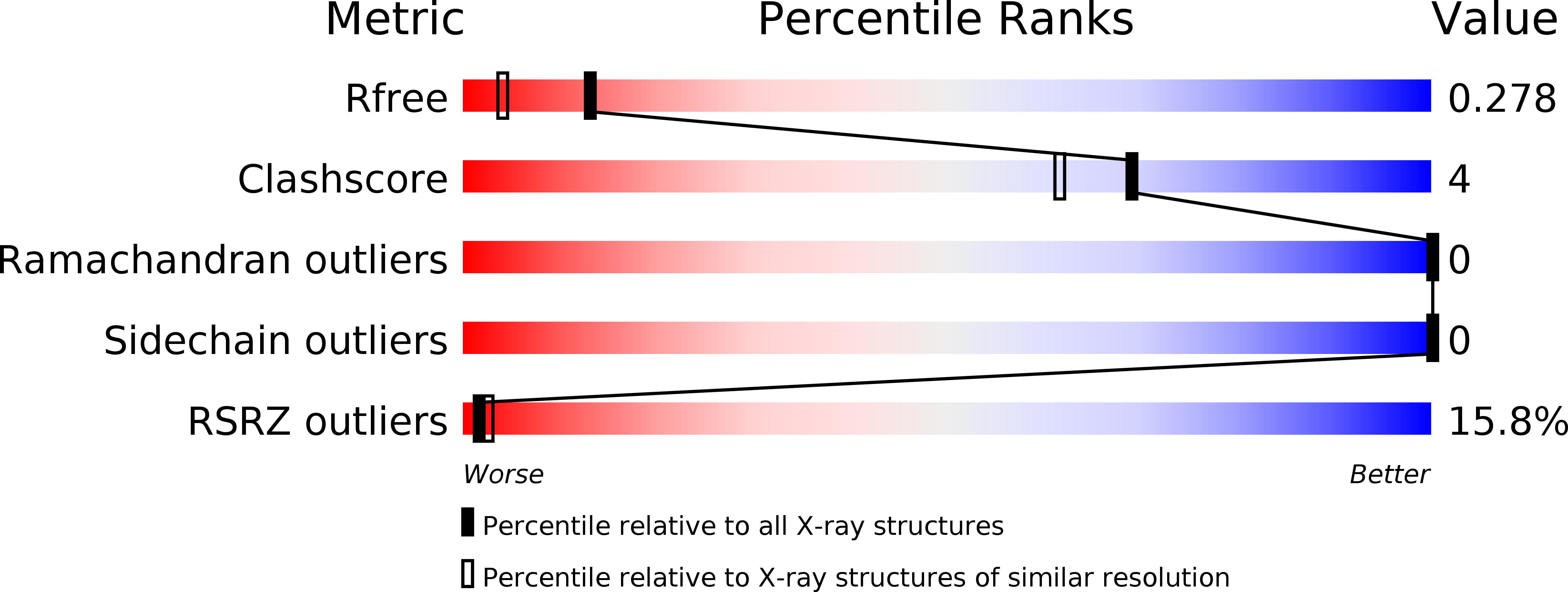
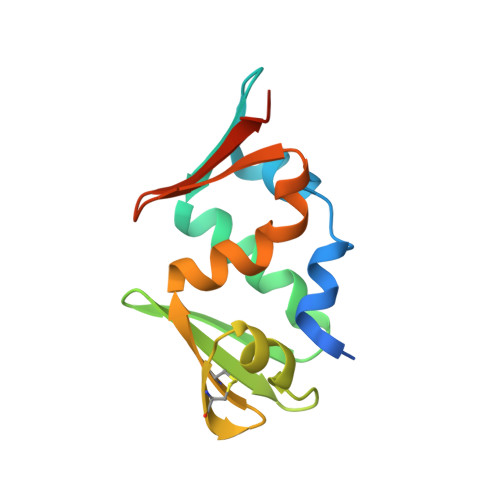
Crystal Structure of Afv1-102, a Protein from the Acidianus Filamentous Virus 1.
Keller, J., Leulliot, N., Collinet, B., Campanacci, V., Cambillau, C., Pranghisvilli, D., Van Tilbeurgh, H.(2009) Protein Sci 18: 845
- PubMed: 19319936
- DOI: https://doi.org/10.1002/pro.79
- Primary Citation of Related Structures:
2WB6 - PubMed Abstract:
Viruses infecting hyperthermophilic archaea have intriguing morphologies and genomic properties. The vast majority of their genes do not have homologs other than in other hyperthermophilic viruses, and the biology of these viruses is poorly understood. As part of a structural genomics project on the proteins of these viruses, we present here the structure of a 102 amino acid protein from acidianus filamentous virus 1 (AFV1-102). The structure shows that it is made of two identical motifs that have poor sequence similarity. Although no function can be proposed from structural analysis, tight binding of the gateway tag peptide in a groove between the two motifs suggests AFV1-102 is involved in protein protein interactions.
Organizational Affiliation:
Institut de Biochimie et de Biophysique Moléculaire et Cellulaire, Université Paris-Sud, IFR115, UMR8619-CNRS, 91405 Orsay, France.