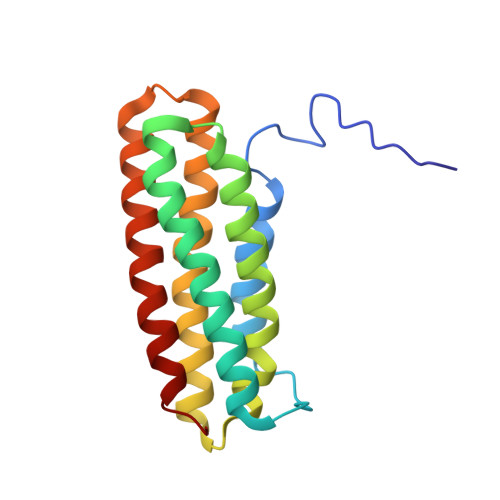
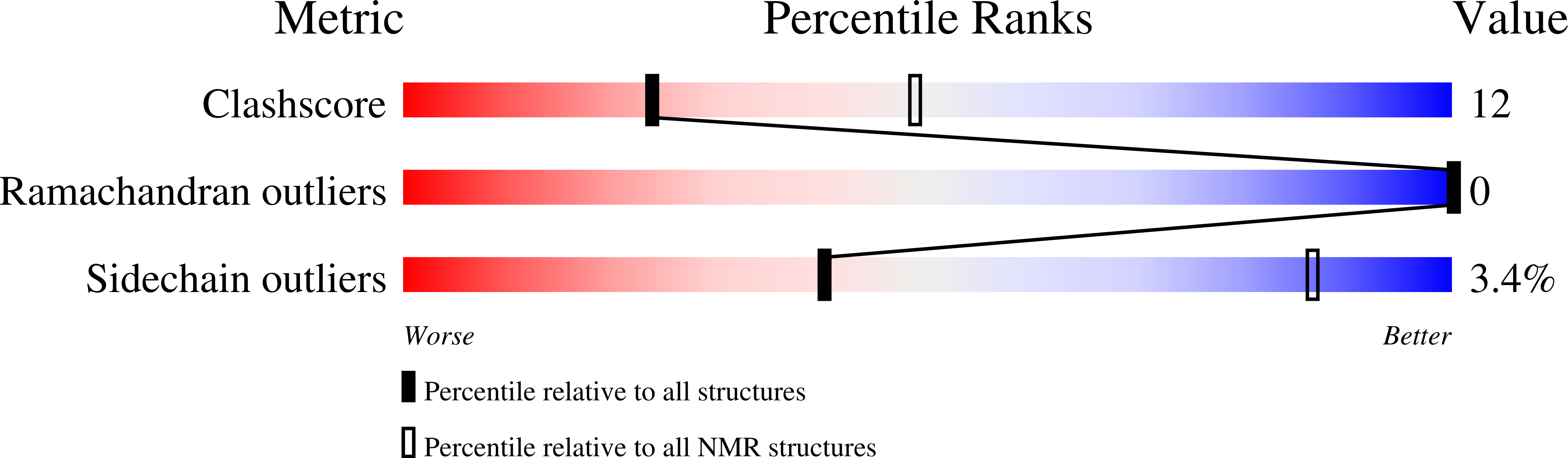The domain structure of talin: Residues 1815-1973 form a five-helix bundle containing a cryptic vinculin-binding site.
Goult, B.T., Gingras, A.R., Bate, N., Barsukov, I.L., Critchley, D.R., Roberts, G.C.(2010) FEBS Lett 584: 2237-2241
- PubMed: 20399778
- DOI: https://doi.org/10.1016/j.febslet.2010.04.028
- Primary Citation of Related Structures:
2KVP - PubMed Abstract:
Talin is a large flexible rod-shaped protein that activates the integrin family of cell adhesion molecules and couples them to cytoskeletal actin. Its rod region consists of a series of helical bundles. Here we show that residues 1815-1973 form a 5-helix bundle, with a topology unique to talin which is optimally suited for formation of a long rod such as talin. This is much more stable than the 4-helix (1843-1973) domain described earlier and as a result its vinculin binding sequence is inaccessible to vinculin at room temperature, with implications for the overall mechanism of the talin-vinculin interaction.
Organizational Affiliation:
Department of Biochemistry, University of Leicester, Leicester, UK.