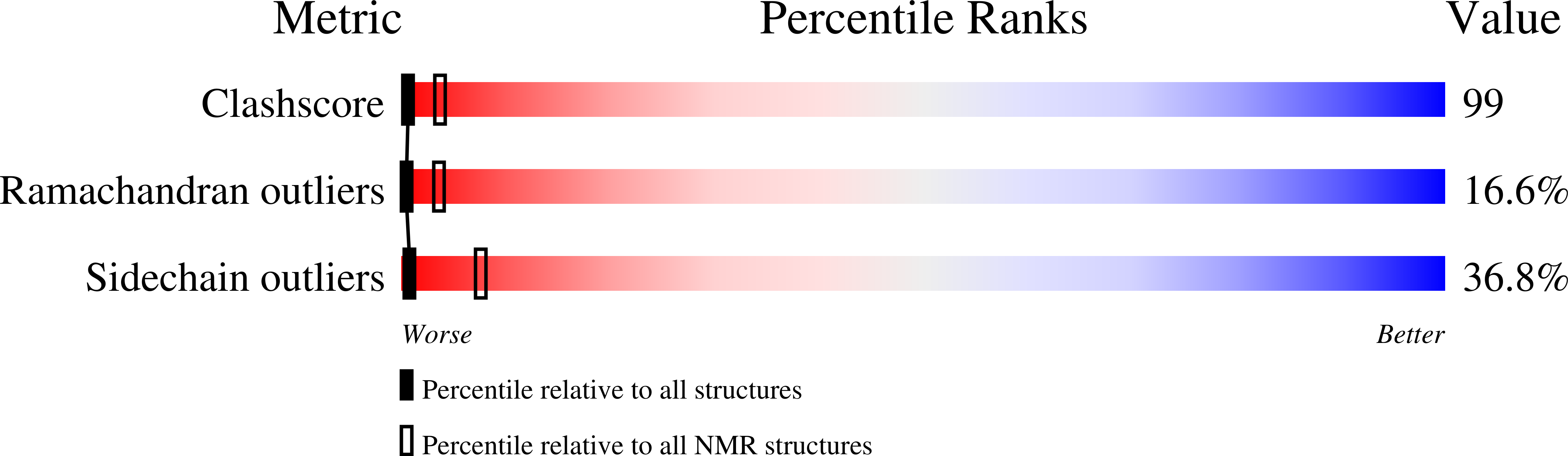NMR structure of Mistic, a membrane-integrating protein for membrane protein expression.
Roosild, T.P., Greenwald, J., Vega, M., Castronovo, S., Riek, R., Choe, S.(2005) Science 307: 1317-1321
- PubMed: 15731457
- DOI: https://doi.org/10.1126/science.1106392
- Primary Citation of Related Structures:
1YGM - PubMed Abstract:
Although structure determination of soluble proteins has become routine, our understanding of membrane proteins has been limited by experimental bottlenecks in obtaining both sufficient yields of protein and ordered crystals. Mistic is an unusual Bacillus subtilis integral membrane protein that folds autonomously into the membrane, bypassing the cellular translocon machinery. Using paramagnetic probes, we determined by nuclear magnetic resonance (NMR) spectroscopy that the protein forms a helical bundle with a surprisingly polar lipid-facing surface. Additional experiments suggest that Mistic can be used for high-level production of other membrane proteins in their native conformations, including many eukaryotic proteins that have previously been intractable to bacterial expression.
Organizational Affiliation:
Structural Biology Laboratory, Salk Institute, San Diego, CA 92037, USA.