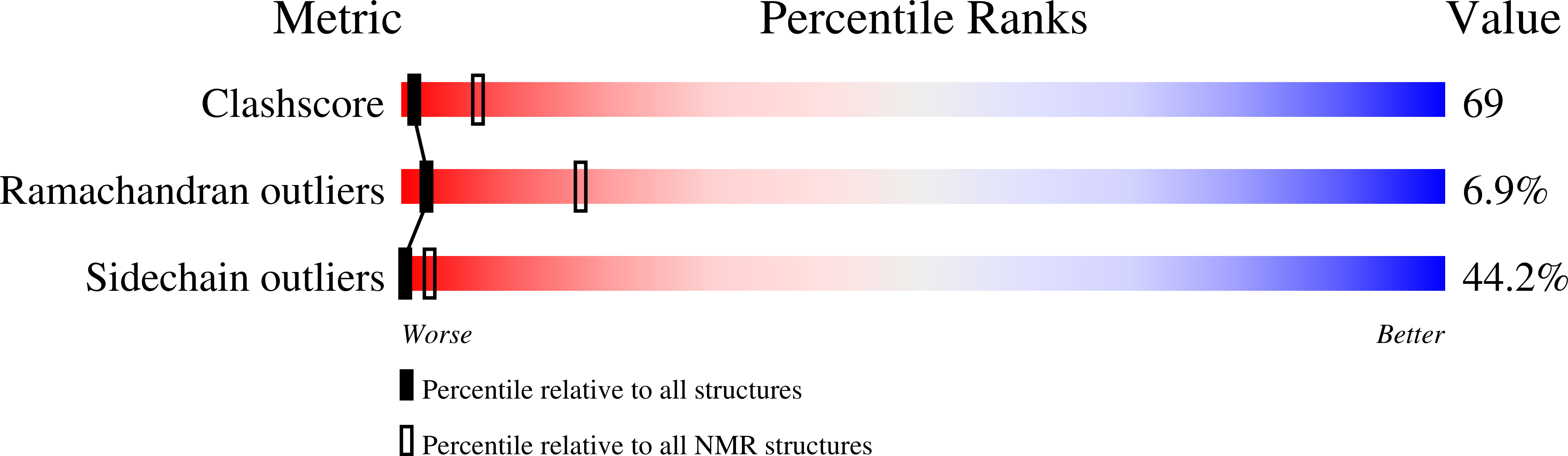Solution structure of the pleckstrin homology domain of Drosophila beta-spectrin.
Zhang, P., Talluri, S., Deng, H., Branton, D., Wagner, G.(1995) Structure 3: 1185-1195
- PubMed: 8591029
- DOI: https://doi.org/10.1016/s0969-2126(01)00254-4
- Primary Citation of Related Structures:
1DRO - PubMed Abstract:
The pleckstrin homology (PH) domain, which is approximately 100 amino acids long, has been found in about 70 proteins involved in signal transduction and cytoskeletal function, a frequency comparable to SH2 (src homology 2) and SH3 domains. PH domains have been shown to bind the beta gamma-subunits of G-proteins and phosphatidylinositol 4,5-bisphosphate (PIP2). It is conceivable that the PH domain of beta-spectrin plays a part in the association of spectrin with the plasma membrane of cells. We have solved the solution structure of the 122-residue PH domain of Drosophila beta-spectrin. The overall fold consists of two antiparallel beta-sheets packing against each other at an angle of approximately 60 degrees to form a beta-sandwich, a two-turn alpha-helix unique to spectrin PH domains, and a four-turn C-terminal alpha-helix. One of the major insertions in beta-spectrin PH domains forms a long, basic surface loop and appears to undergo slow conformational exchange in solution. This loop shows big spectral changes upon addition of D-myo-inositol 1,4,5-trisphosphate (IP3). We propose that the groove at the outer surface of the second beta-sheet is an important site of association with other proteins. This site and the possible lipid-binding site can serve to localize the spectrin network under the plasma membrane. More generally, it has to be considered that the common fold observed for the PH domain structures solved so far does not necessarily mean that all PH domains have similar functions. In fact, the residues constituting potential binding sites for ligands or other proteins are only slightly conserved between different PH domains.
Organizational Affiliation:
Committee on Higher Degrees in Biophysics, Harvard University, Boston, MA 02115, USA.