A novel accessory protein stabilizes the capsid of two actinobacteriophages
Podgorski, J.M., Podgorski, J., Gosselin, S., Abad, L., Jacobs-Sera, D., Brown, C., Hatfull, G., Gogarten, P., Luque, A., White, S.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Major capsid protein | 403 | Mycobacterium phage Adjutor | Mutation(s): 0 | 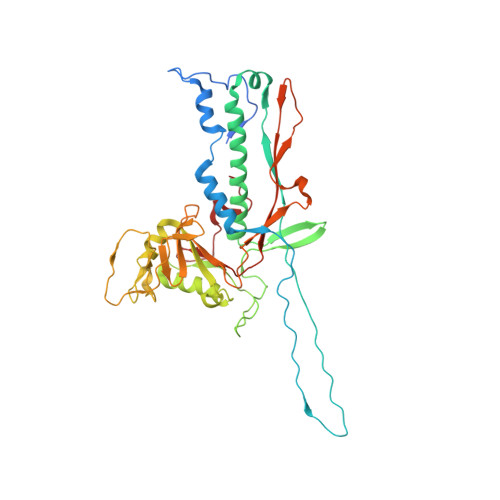 | |
UniProt | |||||
Find proteins for B2ZNR4 (Mycobacterium phage Adjutor) Explore B2ZNR4 Go to UniProtKB: B2ZNR4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B2ZNR4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| gp_16 (Minor Capsid Protein) | 139 | Mycobacterium phage Adjutor | Mutation(s): 0 | 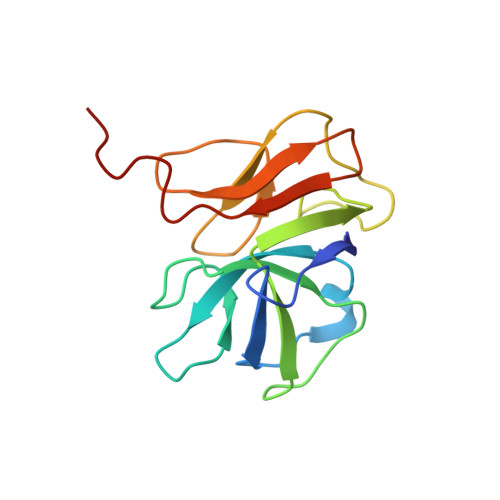 | |
UniProt | |||||
Find proteins for B2ZNR3 (Mycobacterium phage Adjutor) Explore B2ZNR3 Go to UniProtKB: B2ZNR3 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B2ZNR3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| HNH endonuclease | K [auth Y], L [auth Z] | 54 | Mycobacterium phage Adjutor | Mutation(s): 0 | 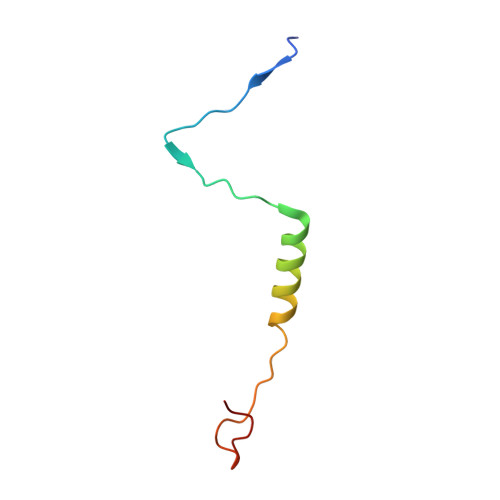 |
UniProt | |||||
Find proteins for B2ZNQ1 (Mycobacterium phage Adjutor) Explore B2ZNQ1 Go to UniProtKB: B2ZNQ1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | B2ZNQ1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1 |
| MODEL REFINEMENT | ISOLDE | 1.3 |
| RECONSTRUCTION | RELION | 3.1.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |