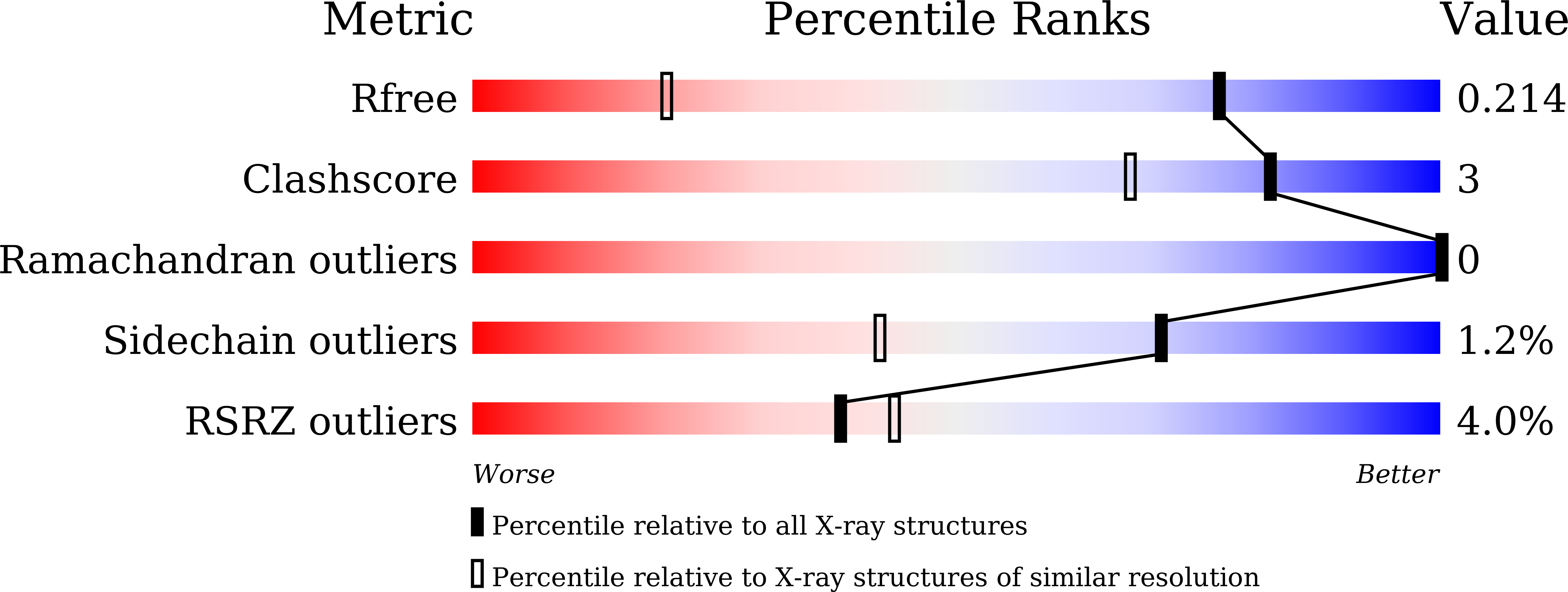
The first crystal structure of CD8 alpha alpha from a cartilaginous fish.
Jia, Z., Feng, J., Dooley, H., Zou, J., Wang, J.(2023) Front Immunol 14: 1156219-1156219
- PubMed: 37122697
- DOI: https://doi.org/10.3389/fimmu.2023.1156219
- Primary Citation of Related Structures:
8HXS - PubMed Abstract:
Cartilaginous fishes are the most evolutionary-distant vertebrates from mammals and possess an immunoglobulin (Ig)- and T cell-mediated adaptive immunity. CD8 is the hallmark receptor of cytotoxic T cells and is required for the formation of T cell receptor-major histocompatibility complex (TCR-MHC) class I complexes. RACE PCR was used to obtain gene sequences. Direct dilution was applied for the refolding of denatured recombinant CD8 protein. Hanging-drop vapor diffusion method was performed for protein crystallization. In this study, CD8α and CD8β orthologues (termed ScCD8α and ScCD8β) were identified in small-spotted catshark ( Scyliorhinus canicula ). Both ScCD8α and ScCD8β possess an extracellular immunoglobulin superfamily (IgSF) V domain as in previously identified CD8 proteins. The genes encoding CD8α and CD8β are tandemly linked in the genomes of all jawed vertebrates studied, suggesting that they were duplicated from a common ancestral gene before the divergence of cartilaginous fishes and other vertebrates. We determined the crystal structure of the ScCD8α ectodomain homodimer at a resolution of 1.35 Å and show that it exhibits the typical topological structure of CD8α from endotherms. As in mammals, the homodimer formation of ScCD8αα relies upon interactions within a hydrophobic core although this differs in position and amino acid composition. Importantly, ScCD8αα shares the canonical cavity required for interaction with peptide-loaded MHC I in mammals. Furthermore, it was found that ScCD8α can co-immunoprecipitate with ScCD8β, indicating that it can form both homodimeric and heterodimeric complexes. Our results expand the current knowledge of vertebrate CD8 dimerization and the interaction between CD8α with p/MHC I from an evolutionary perspective.
Organizational Affiliation:
Key Laboratory of Exploration and Utilization of Aquatic Genetic Resources, Ministry of Education, Shanghai Ocean University, Shanghai, China.